CARVEDILOL Film-coated tablet Ref.[51669] Active ingredients: Carvedilol
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
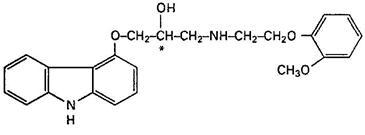
Carvedilol is a nonselective β-adrenergic blocking agent with α1-blocking activity. It is (±)1(Carbazol-4-yloxy)3[[2-(o-methoxyphenoxy)ethyl]amino]-2-propanol. Carvedilol is a racemic mixture with the following structure:
Carvedilol tablets, USP are white, oval, film-coated tablets containing 3.125 mg, 6.25 mg, 12.5 mg, or 25 mg of carvedilol. Inactive ingredients consist of lactose monohydrate, colloidal silicon dioxide, crospovidone, povidone, sucrose, magnesium stearate, polyethylene glycol 400, polysorbate 80, titanium dioxide, and hypromellose.
Carvedilol USP is a white to off-white powder with a molecular weight of 406.5 and a molecular formula of C24H26N2O4. It is freely soluble in dimethylsulfoxide; soluble in methylene chloride and methanol; sparingly soluble in 95% ethanol and isopropanol; slightly soluble in ethyl ether; and practically insoluble in water, gastric fluid (simulated, TS, pH 1.1), and intestinal fluid (simulated, TS without pancreatin, pH 7.5).
Meets USP Dissolution Test 2.
| Dosage Forms and Strengths |
|---|
|
Carvedilol tablets, USP 3.125 mg are white to off-white, oval shaped, film-coated tablets debossed with ‘E’ on one side and ‘01’ on the other side. The 6.25 mg are white to off-white, oval shaped, film-coated tablets debossed with ‘E’ on one side and ‘02’ on the other side. The 12.5 mg are white to off-white, oval shaped, film-coated tablets debossed with ‘E’ on one side and ‘03’ on the other side. The 25 mg are white to off-white, oval shaped, film-coated tablets debossed with ‘E’ on one side and ‘04’ on the other side. |
| How Supplied |
|---|
|
Carvedilol Tablets USP, 3.125 mg are white to off-white, oval shaped, film-coated tablets debossed with ‘E’ on one side and ‘01’ on the other side. Bottles of 100 NDC 65862-142-01 Carvedilol Tablets USP, 6.25 mg are white to off-white, oval shaped, film-coated tablets debossed with ‘E’ on one side and ‘02’ on the other side. Bottles of 100 NDC 65862-143-01 Carvedilol Tablets USP, 12.5 mg are white to off-white, oval shaped, film-coated tablets debossed with ‘E’ on one side and ‘03’ on the other side. Bottles of 100 NDC 65862-144-01 Carvedilol Tablets USP, 25 mg are white to off-white, oval shaped, film-coated tablets debossed with ‘E’ on one side and ‘04’ on the other side. Bottles of 100 NDC 65862-145-01 Distributed by: Aurobindo Pharma USA, Inc., 279 Princeton-Hightstown Road, East Windsor, NJ 08520 Manufactured by: Aurobindo Pharma Limited, Hyderabad-500 032, India |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.