COPALIA HCT Film-coated tablet Ref.[107981] Active ingredients: Amlodipine Hydrochlorothiazide Valsartan Valsartan, Amlodipine and Hydrochlorothiazide
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
4.3. Contraindications
- Hypersensitivity to the active substances, to other sulphonamide derivatives, to dihydropyridine derivatives, or to any of the excipients listed in section 6.1.
- Second and third trimesters of pregnancy (see sections 4.4 and 4.6).
- Hepatic impairment, biliary cirrhosis or cholestasis.
- Severe renal impairment (GFR <30 ml/min/1.73 m²), anuria and patients undergoing dialysis.
- Concomitant use of Copalia HCT with aliskiren-containing products in patients with diabetes mellitus or renal impairment (GFR <60 ml/min/1.73 m²) (see sections 4.5 and 5.1).
- Refractory hypokalaemia, hyponatraemia, hypercalcaemia, and symptomatic hyperuricaemia.
- Severe hypotension.
- Shock (including cardiogenic shock).
- Obstruction of the outflow tract of the left ventricle (e.g. hypertrophic obstructive cardiomyopathy and high grade aortic stenosis).
- Haemodynamically unstable heart failure after acute myocardial infarction.
4.4. Special warnings and precautions for use
The safety and efficacy of amlodipine in hypertensive crisis have not been established.
Sodium- and/or volume-depleted patients
Excessive hypotension, including orthostatic hypotension, was seen in 1.7% of patients treated with the maximum dose of Copalia HCT (10 mg/320 mg/25 mg) compared to 1.8% of valsartan/hydrochlorothiazide (320 mg/25 mg) patients, 0.4% of amlodipine/valsartan (10 mg/320 mg) patients, and 0.2% of hydrochlorothiazide/amlodipine (25 mg/10 mg) patients in a controlled trial in patients with moderate to severe uncomplicated hypertension.
In sodium-depleted and/or volume-depleted patients, such as those receiving high doses of diuretics, symptomatic hypotension may occur after initiation of treatment with Copalia HCT. Copalia HCT should be used only after correction of any pre-existing sodium and/or volume depletion.
If excessive hypotension occurs with Copalia HCT, the patient should be placed in the supine position and, if necessary, given an intravenous infusion of normal saline. Treatment can be continued once blood pressure has been stabilised.
Serum electrolyte changes
Amlodipine/valsartan/hydrochlorothiazide
In the controlled trial of Copalia HCT, the counteracting effects of valsartan 320 mg and hydrochlorothiazide 25 mg on serum potassium approximately balanced each other in many patients. In other patients, one or the other effect may be dominant. Periodic determinations of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals. Periodic determination of serum electrolytes and potassium in particular should be performed at appropriate intervals to detect possible electrolyte imbalance, especially in patients with other risk factors such as impaired renal function, treatment with other medicinal products or history of prior electrolyte imbalances.
Valsartan
Concomitant use with potassium supplements, potassium-sparing diuretics, salt substitutes containing potassium, or other medicinal products that may increase potassium levels (heparin, etc.) is not recommended. Monitoring of potassium should be undertaken as appropriate.
Hydrochlorothiazide
Treatment with Copalia HCT should only start after correction of hypokalaemia and any coexisting hypomagnesaemia. Thiazide diuretics can precipitate new onset hypokalaemia or exacerbate preexisting hypokalaemia. Thiazide diuretics should be administered with caution in patients with conditions involving enhanced potassium loss, for example salt-losing nephropathies and prerenal (cardiogenic) impairment of kidney function. If hypokalaemia develops during hydrochlorothiazide therapy, Copalia HCT should be discontinued until stable correction of the potassium balance.
Thiazide diuretics can precipitate new onset hyponatraemia and hypochloroaemic alkalosis or exacerbate pre-existing hyponatraemia. Hyponatraemia, accompanied by neurological symptoms (nausea, progressive disorientation, apathy) has been observed. Treatment with hydrochlorothiazide should only be started after correction of pre-existing hyponatraemia. In case severe or rapid hyponatraemia develops during Copalia HCT therapy, the treatment should be discontinued until normalisation of natraemia.
All patients receiving thiazide diuretics should be periodically monitored for imbalances in electrolytes, particularly potassium, sodium and magnesium.
Renal impairment
Thiazide diuretics may precipitate azotaemia in patients with chronic kidney disease. When Copalia HCT is used in patients with renal impairment periodic monitoring of serum electrolytes (including potassium), creatinine and uric acid serum levels is recommended. Copalia HCT is contraindicated in patients with severe renal impairment, anuria or undergoing dialysis (see section 4.3).
No dose adjustment of Copalia HCT is required for patients with mild to moderate renal impairment (GFR ≥30 ml/min/1.73 m²).
Renal artery stenosis
Copalia HCT should be used with caution to treat hypertension in patients with unilateral or bilateral renal artery stenosis or stenosis to a solitary kidney since blood urea and serum creatinine may increase in such patients.
Kidney transplantation
To date there is no experience of the safe use of Copalia HCT in patients who have had a recent kidney transplantation.
Hepatic impairment
Valsartan is mostly eliminated unchanged via the bile. The half-life of amlodipine is prolonged and AUC values are higher in patients with impaired liver function; dose recommendations have not been established. In patients with mild to moderate hepatic impairment without cholestasis, the maximum recommended dose is 80 mg valsartan, and therefore, Copalia HCT is not suitable in this group of patients (see sections 4.2, 4.3 and 5.2).
Angioedema
Angioedema, including swelling of the larynx and glottis, causing airway obstruction and/or swelling of the face, lips, pharynx, and/or tongue, has been reported in patients treated with valsartan. Some of these patients previously experienced angioedema with other medicinal products including ACE inhibitors. Copalia HCT should be discontinued immediately in patients who develop angioedema and should not be re-administered.
Heart failure and coronary artery disease/post-myocardial infarction
As a consequence of the inhibition of the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients with severe heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with ACE inhibitors and angiotensin receptor antagonists has been associated with oliguria and/or progressive azotaemia and (rarely) with acute renal failure and/or death. Similar outcomes have been reported with valsartan. Evaluation of patients with heart failure or post-myocardial infarction should always include assessment of renal function.
In a long-term, placebo-controlled study (PRAISE-2) of amlodipine in patients with NYHA (New York Heart Association Classification) III and IV heart failure of non-ischaemic aetiology, amlodipine was associated with increased reports of pulmonary oedema despite no significant difference in the incidence of worsening heart failure as compared to placebo.
Calcium channel blockers, including amlodipine, should be used with caution in patients with congestive heart failure, as they may increase the risk of future cardiovascular events and mortality.
Caution is advised in patients with heart failure and coronary artery disease, particularly at the maximum dose of Copalia HCT, 10 mg/320 mg/25 mg, since available data in these patient populations is limited.
Aortic and mitral valve stenosis
As with all other vasodilators, special caution is indicated in patients with mitral stenosis or significant aortic stenosis that is not high grade.
Pregnancy
Angiotensin II Receptor Antagonists (AIIRAs) should not be initiated during pregnancy. Unless continued AIIRA therapy is considered essential, patients planning pregnancy should be changed to alternative antihypertensive treatments which have an established safety profile for use in pregnancy. When pregnancy is diagnosed, treatment with AIIRAs should be stopped immediately, and, if appropriate, alternative therapy should be started (see sections 4.3 and 4.6).
Primary hyperaldosteronism
Patients with primary hyperaldosteronism should not be treated with the angiotensin II antagonist valsartan as their renin-angiotensin system is not activated. Therefore, Copalia HCT is not recommended in this population.
Systemic lupus erythematosus
Thiazide diuretics, including hydrochlorothiazide, have been reported to exacerbate or activate systemic lupus erythematosus.
Other metabolic disturbances
Thiazide diuretics, including hydrochlorothiazide, may alter glucose tolerance and raise serum levels of cholesterol, triglycerides and uric acid. In diabetic patients dosage adjustments of insulin or oral hypoglycaemic agents may be required.
Due to the hydrochlorothiazide component, Copalia HCT is contraindicated in symptomatic hyperuricaemia. Hydrochlorothiazide may raise the serum uric acid level due to reduced clearance of uric acid and may cause or exacerbate hyperuricaemia as well as precipitate gout in susceptible patients.
Thiazides reduce urinary calcium excretion and may cause intermittant and slight elevation of serum calcium in the absence of known disorders of calcium metabolism. Copalia HCT is contraindicated in patients with hypercalcaemia and should only be used after correction of any pre-existing hypercalcaemia. Copalia HCT should be discontinued if hypercalcaemia develops during treatment. Serum levels of calcium should be periodically monitored during treatment with thiazides. Marked hypercalcaemia may be evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for parathyroid function.
Photosensitivity
Cases of photosensitivity reactions have been reported with thiazide diuretics (see section 4.8). If photosensitivity reaction occurs during treatment with Copalia HCT, it is recommended to stop the treatment. If a readministration of the diuretic is deemed necessary, it is recommended to protect exposed areas to the sun or to artificial UVA.
Choroidal effusion, acute myopia and secondary acute angle-closure glaucoma
Hydrochlorothiazide, a sulphonamide, has been associated with an idiosyncratic reaction resulting in choroidal effusion with visual field defect, acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to a week of treatment initiation. Untreated acute-angle closure glaucoma can lead to permanent vision loss.
The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatment may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle closure glaucoma may include a history of sulphonamide or penicillin allergy.
General
Caution should be exercised in patients who have shown prior hypersensitivity to other angiotensin II receptor antagonists. Hypersensitivity reactions to hydrochlorothiazide are more likely in patients with allergy and asthma.
Elderly (age 65 years or over)
Caution, including more frequent monitoring of blood pressure, is recommended in elderly patients, particularly at the maximum dose of Copalia HCT, 10 mg/320 mg/25 mg, since available data in this patient population are limited.
Dual blockade of the renin-angiotensin-aldosterone system (RAAS)
There is evidence that the concomitant use of ACE inhibitors, ARBs or aliskiren increases the risk of hypotension, hyperkalaemia, and decreased renal function (including acute renal failure). Dual blockade of RAAS through the combined use of ACE inhibitors, ARBs or aliskiren is therefore not recommended (see sections 4.5 and 5.1).
If dual blockade therapy is considered absolutely necessary, this should only occur under specialist supervision and subject to frequent close monitoring of renal function, electrolytes and blood pressure. ACE inhibitors and ARBs should not be used concomitantly in patients with diabetic nephropathy.
Non-melanoma skin cancer
An increased risk of non-melanoma skin cancer (NMSC) [basal cell carcinoma (BCC) and squamous cell carcinoma (SCC)] with increasing cumulative dose of hydrochlorothiazide exposure has been observed in two epidemiological studies based on the Danish National Cancer Registry. Photosensitising actions of hydrochlorothiazide could act as a possible mechanism for NMSC.
Patients taking hydrochlorothiazide should be informed of the risk of NMSC and advised to regularly check their skin for any new lesions and promptly report any suspicious skin lesions. Possible preventive measures such as limited exposure to sunlight and UV rays and, in case of exposure, adequate protection should be advised to the patients in order to minimise the risk of skin cancer. Suspicious skin lesions should be promptly examined potentially including histological examinations of biopsies. The use of hydrochlorothiazide may also need to be reconsidered in patients who have experienced previous NMSC (see also section 4.8).
Acute respiratory toxicity
Very rare severe cases of acute respiratory toxicity, including acute respiratory distress syndrome (ARDS), have been reported after taking hydrochlorothiazide. Pulmonary oedema typically develops within minutes to hours after hydrochlorothiazide intake. At the onset, symptoms include dyspnoea, fever, pulmonary deterioration and hypotension. If diagnosis of ARDS is suspected, Copalia HCT should be withdrawn, and appropriate treatment given. Hydrochlorothiazide should not be administered to patients who previously experienced ARDS following hydrochlorothiazide intake.
4.5. Interaction with other medicinal products and other forms of interaction
No formal interaction studies with other medicinal products have been performed with Copalia HCT. Thus, only information on interactions with other medicinal products that are known for the individual active substances is provided in this section.
However, it is important to take into account that Copalia HCT may increase the hypotensive effect of other antihypertensive agents.
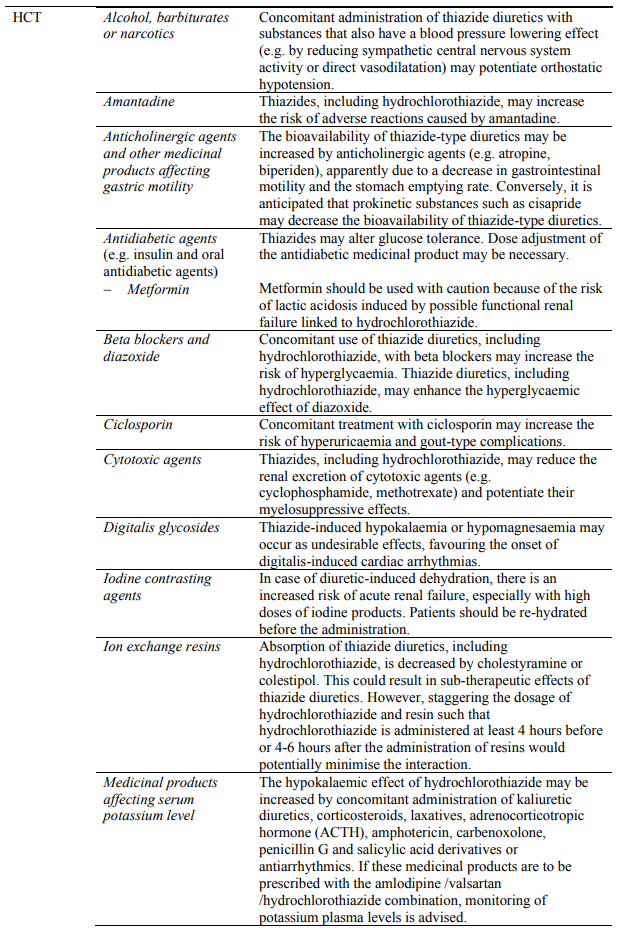
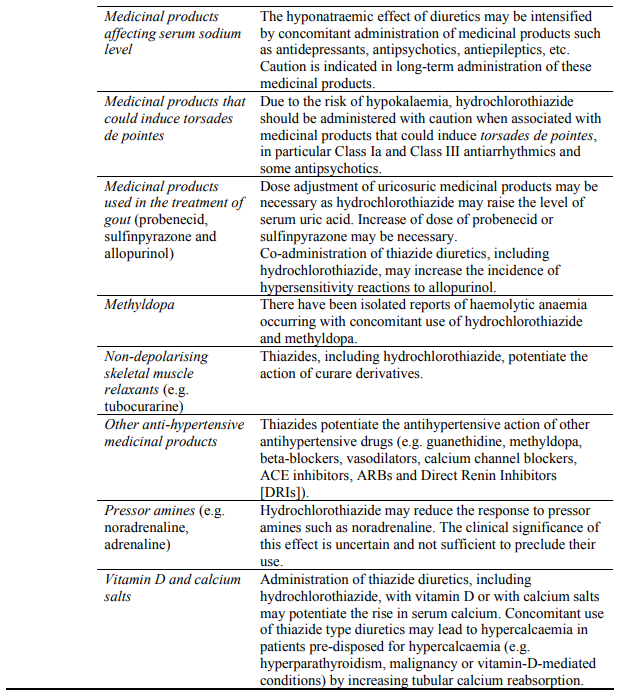
Concomitant use not recommended:
Caution required with concomitant use:
Dual blockade of the RAAS with ARBs, ACE inhibitors or aliskiren
Clinical trial data have shown that dual blockade of the RAAS through the combined use of ACE inhibitors, ARBs or aliskiren is associated with a higher frequency of adverse events such as hypotension, hyperkalaemia and decreased renal function (including acute renal failure) compared to the use of a single RAAS-acting agent (see sections 4.3, 4.4 and 5.1).
4.6. Fertility, pregnancy and lactation
Pregnancy
Amlodipine
The safety of amlodipine in human pregnancy has not been established. In animal studies, reproductive toxicity was observed at high doses (see section 5.3). Use in pregnancy is only recommended when there is no safer alternative and when the disease itself carries greater risk for the mother and foetus.
Valsartan
The use of Angiotensin II Receptor Antagonists (AIIRAs) is not recommended during the first trimester of pregnancy (see section 4.4). The use of AIIRAs is contraindicated during the second and third trimesters of pregnancy (see sections 4.3 and 4.4).
Epidemiological evidence regarding the risk of teratogenicity following exposure to ACE inhibitors during the first trimester of pregnancy has not been conclusive; however a small increase in risk cannot be excluded. Whilst there is no controlled epidemiological data on the risk with Angiotensin II Receptor Antagonists (AIIRAs), similar risks may exist for this class of drugs. Unless continued AIIRA therapy is considered essential, patients planning pregnancy should be changed to alternative antihypertensive treatments which have an established safety profile for use in pregnancy. When pregnancy is diagnosed, treatment with AIIRAs should be stopped immediately, and if appropriate, alternative therapy should be started.
Exposure to AIIRAs therapy during the second and third trimesters is known to induce human foetotoxicity (decreased renal function, oligohydramnios, skull ossification retardation) and neonatal toxicity (renal failure, hypotension, hyperkalaemia) (see section 5.3).
Should exposure to AIIRAs have occurred from the second trimester of pregnancy, ultrasound check of renal function and skull is recommended.
Infants whose mothers have taken AIIRAs should be closely observed for hypotension (see sections 4.3 and 4.4).
Hydrochlorothiazide
There is limited experience with hydrochlorothiazide during pregnancy, especially during the first trimester. Animal studies are insufficient.
Hydrochlorothiazide crosses the placenta. Based on the pharmacological mechanism of action of hydrochlorothiazide, its use during the second and third trimester may compromise foeto-placental perfusion and may cause foetal and neonatal effects like icterus, disturbance of electrolyte balance and thrombocytopenia.
Amlodipine/valsartan/hydrochlorothiazide
There is no experience on the use of Copalia HCT in pregnant women. Based on the existing data with the components, the use of Copalia HCT is not recommended during first trimester and contraindicated during the second and third trimester of pregnancy (see sections 4.3 and 4.4).
Breast-feeding
Amlodipine is excreted in human milk. The proportion of the maternal dose received by the infant has been estimated with an interquartile range of 3-7%, with a maximum of 15%. The effect of amlodipine on infants is unknown. No information is available regarding the use of valsartan during breastfeeding. Hydrochlorothiazide is excreted in human milk in small amounts. Thiazides in high doses causing intense diuresis can inhibit milk production. The use of Copalia HCT during breast-feeding is not recommended. If Copalia HCT is used during breast-feeding, doses should be kept as low as possible. Alternative treatments with better established safety profiles during breast-feeding are preferable, especially while nursing a newborn or preterm infant.
Fertility
There are no clinical studies on fertility with Copalia HCT.
Valsartan
Valsartan had no adverse effects on the reproductive performance of male or female rats at oral doses up to 200 mg/kg/day. This dose is 6 times the maximum recommended human dose on a mg/m² basis (calculations assume an oral dose of 320 mg/day and a 60-kg patient).
Amlodipine
Reversible biochemical changes in the head of spermatozoa have been reported in some patients treated by calcium channel blockers. Clinical data are insufficient regarding the potential effect of amlodipine on fertility. In one rat study, adverse effects were found on male fertility (see section 5.3).
4.7. Effects on ability to drive and use machines
Patients taking Copalia HCT and driving vehicles or using machines should take into account that dizziness or weariness may occasionally occur.
Amlodipine can have mild or moderate influence on the ability to drive and use machines. If patients taking Copalia HCT suffer from dizziness, headache, fatigue or nausea the ability to react may be impaired.
4.8. Undesirable effects
The safety profile of Copalia HCT presented below is based on clinical studies performed with Copalia HCT and the known safety profile of the individual components amlodipine, valsartan and hydrochlorothiazide.
Summary of the safety profile
The safety of Copalia HCT has been evaluated at its maximum dose of 10 mg/320 mg/25 mg in one controlled short-term (8 weeks) clinical study with 2,271 patients, 582 of whom received valsartan in combination with amlodipine and hydrochlorothiazide. Adverse reactions were generally mild and transient in nature and only infrequently required discontinuation of therapy. In this active controlled clinical trial, the most common reasons for discontinuation of therapy with Copalia HCT were dizziness and hypotension (0.7%).
In the 8-week controlled clinical study, no significant new or unexpected adverse reactions were observed with triple therapy treatment compared to the known effects of the monotherapy or dual therapy components.
In the 8-week controlled clinical study, changes in laboratory parameters observed with the combination of Copalia HCT were minor and consistent with the pharmacological mechanism of action of the monotherapy agents. The presence of valsartan in the triple combination attenuated the hypokalaemic effect of hydrochlorothiazide.
Tabulated list of adverse reactions
The following adverse reactions, listed by MedDRA System Organ Class and frequency, concern Copalia HCT (amlodipine/valsartan/HCT) and amlodipine, valsartan and HCT individually. Very common: ≥1/10; common: ≥1/100 to <1/10; uncommon: ≥1/1,000 to <1/100; rare: ≥1/10,000 to <1/1,000; very rare: <1/10,000, not known (cannot be estimated from the available data).
| MedDRA System Organ Class | Adverse reactions | Frequency | |||
|---|---|---|---|---|---|
| Copalia HCT | Amlodipine | Valsartan | HCT | ||
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | Non-melanoma skin cancer (Basal cell carcinoma and Squamous cell carcinoma) | -- | -- | -- | Not known |
| Blood and lymphatic system disorders | Agranulocytosis, bone marrow failure | -- | -- | -- | Very rare |
| Haemoglobin and haematocrit decreased | -- | -- | Not known | -- | |
| Haemolytic anaemia | -- | -- | -- | Very rare | |
| Leukopenia | -- | Very rare | -- | Very rare | |
| Neutropenia | -- | -- | Not known | -- | |
| Thrombocytopenia, sometimes with purpura | -- | Very rare | Not known | Rare | |
| Aplastic anaemia | -- | -- | -- | Not known | |
| Immune system disorders | Hypersensitivity | -- | Very rare | Not known | Very rare |
| Metabolism and nutrition disorders | Anorexia | Uncommon | -- | -- | -- |
| Hypercalcaemia | Uncommon | -- | -- | Rare | |
| Hyperglycaemia | -- | Very rare | -- | Rare | |
| Hyperlipidaemia | Uncommon | -- | -- | -- | |
| Hyperuricaemia | Uncommon | -- | -- | Common | |
| Hypochloraemic alkalosis | -- | -- | -- | Very rare | |
| Hypokalaemia | Common | -- | -- | Very common | |
| Hypomagnesaemia | -- | -- | -- | Common | |
| Hyponatraemia | Uncommon | -- | -- | Common | |
| Worsening of diabetic metabolic state | -- | -- | -- | Rare | |
| Psychiatric disorders | Depression | -- | Uncommon | -- | Rare |
| Insomnia/sleep disorders | Uncommon | Uncommon | -- | Rare | |
| Mood swings | -- | Uncommon | -- | -- | |
| Confusion | -- | Rare | -- | -- | |
| Nervous system disorders | Coordination abnormal | Uncommon | -- | -- | -- |
| Dizziness | Common | Common | -- | Rare | |
| Dizziness postural, dizziness exertional | Uncommon | -- | -- | -- | |
| Dysgeusia | Uncommon | Uncommon | -- | -- | |
| Extrapyramidal syndrome | -- | Not known | -- | -- | |
| Headache | Common | Common | -- | Rare | |
| Hypertonia | -- | Very rare | -- | -- | |
| Lethargy | Uncommon | -- | -- | -- | |
| Paraesthesia | Uncommon | Uncommon | -- | Rare | |
| Peripheral neuropathy, neuropathy | Uncommon | Very rare | -- | -- | |
| Somnolence | Uncommon | Common | -- | -- | |
| Syncope | Uncommon | Uncommon | -- | -- | |
| Tremor | -- | Uncommon | -- | -- | |
| Hypoesthesia | -- | Uncommon | -- | -- | |
| Eye disorders | Acute angle-closure glaucoma | -- | -- | -- | Not known |
| Visual disturbance | -- | Uncommon | -- | -- | |
| Visual impairment | Uncommon | Uncommon | -- | Rare | |
| Choroidal effusion | -- | -- | -- | Not Known | |
| Ear and labyrinth disorders | Tinnitus | -- | Uncommon | -- | -- |
| Vertigo | Uncommon | -- | Uncommon | -- | |
| Cardiac disorders | Palpitations | -- | Common | -- | -- |
| Tachycardia | Uncommon | -- | -- | -- | |
| Arrhythmias (including bradycardia, ventricular tachycardia, and atrial fibrillation) | -- | Very rare | -- | Rare | |
| Myocardial infarction | -- | Very rare | -- | -- | |
| Vascular disorders | Flushing | -- | Common | -- | -- |
| Hypotension | Common | Uncommon | -- | -- | |
| Orthostatic hypotension | Uncommon | -- | -- | Common | |
| Phlebitis, thrombophlebitis | Uncommon | -- | -- | -- | |
| Vasculitis | -- | Very rare | Not known | -- | |
| Respiratory, thoracic and mediastinal disorders | Cough | Uncommon | Very rare | Uncommon | -- |
| Dyspnoea | Uncommon | Uncommon | -- | -- | |
| Acute respiratory distress syndrome (ARDS) (see section 4.4) | -- | -- | -- | Very rare | |
| Respiratory distress, pulmonary oedema, pneumonitis | -- | -- | -- | Very rare | |
| Rhinitis | -- | Uncommon | -- | -- | |
| Throat irritation | Uncommon | -- | -- | -- | |
| Gastrointestinal disorders | Abdominal discomfort, abdominal pain upper | Uncommon | Common | Uncommon | Rare |
| Breath odour | Uncommon | -- | -- | -- | |
| Change of bowel habit | -- | Uncommon | -- | -- | |
| Constipation | -- | -- | -- | Rare | |
| Decreased appetite | -- | -- | -- | Common | |
| Diarrhoea | Uncommon | Uncommon | -- | Rare | |
| Dry mouth | Uncommon | Uncommon | -- | -- | |
| Dyspepsia | Common | Uncommon | -- | -- | |
| Gastritis | -- | Very rare | -- | -- | |
| Gingival hyperplasia | -- | Very rare | -- | -- | |
| Nausea | Uncommon | Common | -- | Common | |
| Pancreatitis | -- | Very rare | -- | Very rare | |
| Vomiting | Uncommon | Uncommon | -- | Common | |
| Hepatobiliary disorders | Liver function test abnormal, including blood bilirubin increase | -- | Very rare** | Not known | -- |
| Hepatitis | -- | Very rare | -- | -- | |
| Intrahepatic cholestasis, jaundice | -- | Very rare | -- | Rare | |
| Skin and subcutaneous tissue disorders | Alopecia | -- | Uncommon | -- | -- |
| Angioedema | -- | Very rare | Not known | -- | |
| Dermatitis bullous | -- | -- | Not known | -- | |
| Cutaneous lupus erythematosus-like reactions, reactivation of cutaneous lupus erythematosus | -- | -- | -- | Very rare | |
| Erythema multiforme | -- | Very rare | -- | Not known | |
| Exanthema | -- | Uncommon | -- | -- | |
| Hyperhidrosis | Uncommon | Uncommon | -- | -- | |
| Photosensitivity reaction* | -- | Very rare | -- | Rare | |
| Pruritus | Uncommon | Uncommon | Not known | -- | |
| Purpura | -- | Uncommon | -- | Rare | |
| Rash | -- | Uncommon | Not known | Common | |
| Skin discoloration | -- | Uncommon | -- | -- | |
| Urticaria and other forms of rash | -- | Very rare | -- | Common | |
| Vasculitis necrotising and toxic epidermal necrolysis | -- | Not known | -- | Very rare | |
| Exfoliative dermatitis | -- | Very rare | -- | -- | |
| Stevens-Johnson syndrome | -- | Very rare | -- | -- | |
| Quincke oedema | -- | Very rare | -- | -- | |
| Musculoskeletal and connective tissue disorders | Arthralgia | -- | Uncommon | -- | -- |
| Back pain | Uncommon | Uncommon | -- | -- | |
| Joint swelling | Uncommon | -- | -- | -- | |
| Muscle spasm | Uncommon | Uncommon | -- | Not known | |
| Muscular weakness | Uncommon | -- | -- | -- | |
| Myalgia | Uncommon | Uncommon | Not known | -- | |
| Pain in extremity | Uncommon | -- | -- | -- | |
| Ankle swelling | -- | Common | -- | -- | |
| Renal and urinary disorders | Blood creatinine increased | Uncommon | -- | Not known | -- |
| Micturition disorder | -- | Uncommon | -- | -- | |
| Nocturia | -- | Uncommon | -- | -- | |
| Pollakiuria | Common | Uncommon | -- | -- | |
| Renal dysfunction | -- | -- | -- | Not known | |
| Acute renal failure | Uncommon | -- | -- | Not known | |
| Renal failure and impairment | -- | -- | Not known | Rare | |
| Reproductive system and breast disorders | Impotence | Uncommon | Uncommon | -- | Common |
| Gynaecomastia | -- | Uncommon | -- | -- | |
| General disorders and administration site conditions | Abasia, gait disturbance | Uncommon | -- | -- | -- |
| Asthenia | Uncommon | Uncommon | -- | Not known | |
| Discomfort, malaise | Uncommon | Uncommon | -- | -- | |
| Fatigue | Common | Common | Uncommon | -- | |
| Non cardiac chest pain | Uncommon | Uncommon | -- | -- | |
| Oedema | Common | Common | -- | -- | |
| Pain | -- | Uncommon | -- | -- | |
| Pyrexia | -- | -- | -- | Not known | |
| Investigations | Lipids increased | -- | -- | -- | Very common |
| Blood urea nitrogen increased | Uncommon | -- | -- | -- | |
| Blood uric acid increased | Uncommon | -- | -- | -- | |
| Glycosuria | -- | -- | -- | Rare | |
| Blood potassium decreased | Uncommon | -- | -- | -- | |
| Blood potassium increased | -- | -- | Not known | -- | |
| Weight increase | Uncommon | Uncommon | -- | -- | |
| Weight decrease | -- | Uncommon | -- | -- | |
* See section 4.4 Photosensitivity
** Mostly consistent with cholestasis
Description of selected adverse reactions
Non-melanoma skin cancer
Based on available data from epidemiological studies, cumulative dose-dependent association between hydrochlorothiazide and NMSC has been observed (see also sections 4.4 and 5.1).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.