DEPO-MEDROL Suspension for injection Ref.[50761] Active ingredients: Methylprednisolone
Source: Medicines and Medical Devices Safety Authority (NZ) Revision Year: 2020 Publisher: Pfizer New Zealand Ltd, PO Box 3998, Auckland, New Zealand, 1140
5.1. Pharmacodynamic properties
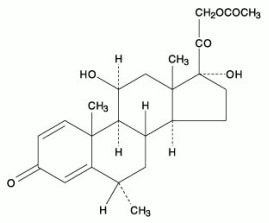
The chemical name for methylprednisolone acetate is pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-11,17-dihydroxy-6-methyl-,(6α,11ß).
The structural formula is shown below:
Molecular formula: C24H32O6
Molecular weight: 416.51
CAS Registry Number: 53-36-1.
Methylprednisolone is an anti-inflammatory steroid. Estimates of the relative potencies of methylprednisolone relative to prednisolone range from 1.13 to 2.1 with an average of 1.5. In general the required daily dose of methylprednisolone can be estimated to be two thirds (or 0.7) the required daily dose of prednisolone. While the effect of parenterally administered methylprednisolone acetate is prolonged, it has the same metabolic and anti-inflammatory actions as orally administered medicine.
Cortisol and its synthetic analogues, such as methylprednisolone acetate, exert their action locally by preventing or suppressing the development of local heat, redness, swelling and tenderness by which inflammation is recognized at the gross level of observation. At the microscopic level, such compounds inhibit not only the early phenomena of the inflammatory process (oedema, fibrin deposition, capillary dilation, migration of phagocytes into the inflamed areas and phagocytic activity), but also the later manifestations (capillary proliferation, fibroblast proliferation, deposition of collagen and still later cicatrisation). These compounds inhibit inflammatory response whether the inciting agent is mechanical, chemical or immunological.
5.2. Pharmacokinetic properties
Absorption
Methylprednisolone acetate is hydrolysed to its active form by serum cholinesterases. The intracellular activity of glucocorticoids results in a clear difference between plasma half-life and pharmacological half-life. Pharmacological activity persists after measurable plasma levels have disappeared.
The duration of anti-inflammatory activity of glucocorticoids approximately equals the duration of hypothalamic-pituitary-adrenal (HPA) axis suppression.
Intramuscular (I.M.) injections of 40 mg/mL give after approximately 7.3 ± 1 hour (Tmax) methylprednisolone serum peaks of 1.48 ± 0.86 mcg/100 mL (Cmax). The half-life is in this case 69.3 hours. After a single I.M. injection of 40 to 80 mg methylprednisolone acetate, duration of HPA axis suppression ranged from 4 to 8 days. An intra-articular injection of 40 mg in both knees (total dose: 80 mg) gives after 4 to 8 hours methylprednisolone peaks of approximately 21.5 mcg/100 mL.
Distribution
After intra-articular administration methylprednisolone acetate diffuses from the joint into systemic circulation over approximately 7 days, as demonstrated by the duration of the HPA axis suppression and by the serum methylprednisolone values.
In man, methylprednisolone forms a weak dissociable bond with albumin and transcortin. Approximately 40 to 90% of the drug is bound.
Biotransformation or Metabolism
Metabolism of methylprednisolone occurs via hepatic routes qualitatively similar to that of cortisol. The major metabolites are 20 beta-hydroxymethylprednisolone and 20 beta-hydroxy-6-alphamethylprednisone. The metabolites are mainly excreted in the urine as glucuronides, sulphates and unconjugated compounds. These conjugation reactions occur principally in the liver and to some extent in the kidney.
5.3. Preclinical safety data
Genotoxicity
Methylprednisolone acetate has not been formally evaluated for genotoxicity. However, methylprednisolone sulfonate, which is structurally similar to methylprednisolone, was not mutagenic in bacteria (Ames test), or in a mammalian cell gene mutation assay using Chinese hamster ovary cells. Methylprednisolone suleptanate did not induce unscheduled DNA synthesis in primary rat hepatocytes. Prednisolone farnesylate, which is also structurally similar to methylprednisolone, was not mutagenic in bacteria, but displayed weak clastogenic activity in vitro in Chinese hamster lung fibroblasts in the presence of metabolic activation.
Carcinogenicity
Methylprednisolone has not been formally evaluated in rodent carcinogenicity studies. Negative results for carcinogenicity have been obtained with various other glucocorticoids including budesonide, prednisolone and triamcinolone acetonide, in mice. However, all three of these compounds were shown to increase the incidence of hepatocellular adenomas and carcinomas after oral administration in a 2-year study in male rats. These tumorigenic effects occurred at doses that are less than the typical clinical doses on a mg/m² basis. Hepatocarcinogenicity is likely to involve an interaction with the glucocorticoid receptor.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.