EMCITATE Dispersible tablet Ref.[114870] Active ingredients: Tiratricol
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Rare Thyroid Therapeutics International AB, Klara Norra Kyrkogata 26, 111 22, Stockholm, Sweden
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: thyroid hormones
ATC code: H03AA04
Mechanism of action
Tiratricol (3,3',5-triiodothyroacetic acid) is a naturally circulating metabolite of active thyroid hormone (T3) with a high degree of structural similarity and follows the same downward degradation pathway (deiodination and conjugation) and elimination via bile and urine. Tiratricol is biologically active, binds with high affinity to the thyroid hormone receptors TRα and TRβ and exerts similar biological effects to T3, although with different tissue specificity. Tiratricol has been demonstrated to be able to enter MCT8 dependent cells without a functioning MCT8 transporter unlike T3 and T4. Tiratricol can thereby replace T3 in MCT8 dependent tissues and restore normal thyroid hormone activity across tissues.
Clinical efficacy and safety
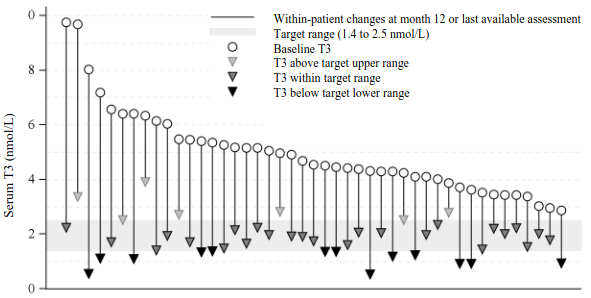
The effect of tiratricol in treatment of patients with MCT8 deficiency was evaluated in a single arm, open label, multicentre study (Triac Trial I) conducted in 46 patients treated for up to 12 months with an individually titrated dose of tiratricol based on serum T3 levels (target range 1.4 to 2.5 nmol/L). The median age of patients enrolled was 7.1 years, with a range from 10 months to 66.8 years. Forty (40) patients were treated for at least 12 months. The median daily maintenance dose administered was 700 micrograms (38.9 micrograms/kg body weight) with a range from 350 micrograms to 2 100 micrograms. Tiratricol treatment reduced the mean serum T3 concentration from 4.97 nmol/L at baseline to 1.82 nmol/L at month 12 (target range 1.4 to 2.5 nmol/L). All 45 patients with post-baseline T3 values presented a decrease from baseline to month 12 or last available assessment. At month 12, or last available assessment, 25 out of 45 patients (56%) attained serum T3 levels within the target range, 13 out of 45 patients (29%) had T3 levels below the target range, and 7 out of 45 patients (16%) had serum T3 levels above the target range. The results for the primary endpoint are presented in Table 4 and Figure 1.
Table 4. Mean change in serum T3 from baseline to month 12 in Triac Trial I (intention-to-treat (ITT) population):
| Variable | N | Baseline mean (SD) | Month 12 mean (SD) | Difference mean [95% CI] | p-value |
|---|---|---|---|---|---|
| Serum T3 (nmol/L) | 45 | 4.97 (1.55) | 1.82 (0.69) | -3.15 [-3.62; -2.68] | <0.0001 |
Figure 1. Serum T3 concentrations at baseline and month 12 in Triac Trial I (ITT population):
Table 5. Other thyroid hormones – analysis of mean change from baseline to month 12 in Triac Trial I (ITT population):
| Variable | N | Baseline mean (SD) | Month 12 mean (SD) | Difference mean [95% CI] | p-value |
|---|---|---|---|---|---|
| TSH (mU/L) | 45 | 2.91 (1.68) | 1.02 (1.14) | -1.89 [-2.39; -1.39] | <0.0001 |
| Free T4 (pmol/L) | 45 | 9.68 (2.96) | 3.39 (1.60) | -6.28 [-7.15; -5.41] | <0.0001 |
| Total T4 (nmol/L) | 45 | 55.96 (12.95) | 24.38 (9.44) | -31.58 [-35.15; -28.01] | <0.0001 |
| rT3 (nmol/L) | 45 | 0.12 (0.10) | 0.04 (0.04) | -0.08 [-0.10; -0.05] | <0.0001 |
In Triac Trial I, the mean body weight for age MCT8 Z score (comparing tiratricol treated MCT8 patients with untreated MCT8 patients) was increased from 0.46 at baseline to 0.96 at month 12 (mean change 0.51; 95% CI: 0.25, 0.76), while the mean body weight for age Z scores (comparing tiratricol treated MCT8 patients with a normal population) increased modestly from -2.85 at baseline to -2.63 at month 12 (mean change 0.22; 95% CI: -0.01, 0.45). Results were similar in patients with or without gastroenteral feeding tube at baseline. In total, 40 out of 45 patients (89%) increased in body weight; 28 out of 45 (62%) had an increase in body weight for age Z score, and 28 out of 36 (78%) had an increase in body weight for age MCT8 Z score.
In patients below 2.5 years of age, based on few individuals, mean body weight for age MCT8 Z score increased from -0.10 at baseline to 0.41 at month 12 (n=3), while the mean body weight for age Z scores increased modestly from -1.65 at baseline to -1.61 at month 12 (n=4).
Mean resting heart rate was reduced from 112.4 bpm at baseline to 103.5 bpm at month 12 (mean change -8.9 bpm; 95% CI: -15.6, -2.3), while the mean heart rate for age Z scores (comparing tiratricol treated MCT8 deficiency patients with a normal population) decreased from 1.72 at baseline to 1.38 at month 12 (mean change -0.33; 95% CI: -0.77, 0.10). In patients with tachycardia at baseline, mean resting heart rate was reduced from 131.4 bpm at baseline to 109.6 bpm at month 12 (mean change -21.9 bpm; 95% CI: -30.0, -13.8), while the mean heart rate for age Z scores decreased from 2.80 at baseline to 1.75 at month 12 (mean change -1.05; 95% CI: -1.55, -0.54). In total, 23 out of 34 patients (67%) had a decrease in resting heart rate. In patients with tachycardia at baseline, 15 out of 16 (94%) had a decrease in resting heart rate.
Mean systolic blood pressure was reduced from 107.1 mmHg at baseline to 103.0 mmHg at month 12 (mean change -4.1 mmHg; 95% CI: -8.1, 0.1). In hypertensive patients, mean systolic blood pressure was reduced from 110.9 mmHg at baseline to 102.5 mmHg at month 12 (mean change -8.4 mmHg; 95% CI: -11.7, -5.0). The percentage of patients with hypertension was reduced from 40% at baseline to 17% at month 12 (p=0.02). In total, 24 out of 35 patients (69%) had a decrease in systolic blood pressure. In patients with hypertension at baseline, 12 out of 12 (100%) had a decrease in systolic blood pressure.
In Triac Trial I, all patients (45 out of 45; 100%) improved in at least one of the variables: body weight, resting heart rate, or systolic blood pressure and 31 out of 45 (69%) improved in at least two of these three variables. In total, 39 out of 45 patients (87%) improved in at least one of the variables: body weight for age MCT8 Z score, resting heart rate Z score, or systolic blood pressure Z score and 21 out of 45 (47%) improved in at least two of these three variables.
The mean number of premature atrial contractions measured by 24-hour ECG decreased from 899.7 PACs/24-hours at baseline to 313.9 PACs/24-hours at month 12 (mean change -586; 95% CI: -955, -217).
Creatinine kinase concentrations increased from 108 U/L at baseline to 160.7 U/L at month 12 (mean change 52.7; 95% CI: 27.3,78.1; p=0.0001).
5.2. Pharmacokinetic properties
Absorption
The absorption of tiratricol following oral dosing is rapid with a median tmax of 0.5 hours following doses between 175 and 1 050 micrograms in fasted healthy volunteers.
Distribution
The in vitro plasma protein binding of tiratricol is high, with protein binding of > 99% in human plasma. The bioavailability of tiratricol (F) was 67 ± 6% suggesting tiratricol is well absorbed from the gastrointestinal (GI) tract.
Biotransformation
Tiratricol is a naturally circulating metabolite of active T3 with a high degree of structural similarity and follows the same metabolic pathway. The major human metabolic pathway of tiratricol is by stepwise deiodination, sulfation and glucuronidation mainly in the liver, similar to T3.
Elimination
Following Cmax, serum concentrations declined in a generally biphasic manner and remained quantifiable until between 3 and 48 hours post dose. The geometric mean t½ was between 13.3–14.0 hours for the 350 microgram and 1 050 microgram doses, respectively. Tiratricol is eliminated through bile and urine.
Linearity
Cmax following treatment with the 175 microgram, 350 microgram, and 1 050 microgram doses (about 2 to 13.5 micrograms/kg bodyweight) increased proportionally with dose, whereas the area under the curve (AUC) increased in a slightly greater than proportional manner with increasing dose.
Pharmacokinetic/pharmacodynamic relationship(s)
In the clinical trial studying the effect of tiratricol in patients with MCT8 deficiency, the dose was individually titrated based on T3 levels.
5.3. Preclinical safety data
No conventional studies of carcinogenic potential have been conducted with tiratricol. Tiratricol was devoid of mutagenic activity when tested in the Ames Salmonella assay and showed no increase in chromosomal aberrations when tested in vitro and in vivo.
Embryofetal development studies showed embryolethality in rabbits and embryolethality and structural myocardial damage in rats. On a mg/body surface area (BSA) dose comparison, the no- observed-adverse-effect-levels (NOAELs) in the rat and rabbit studies were slightly lower and slightly higher, respectively, than the highest clinical dose in adult patients.
No effects on mating ability or fertility were observed in a study in male and female rats administered high and otherwise toxic doses of tiratricol.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.