FASENRA Solution for injection Ref.[109450] Active ingredients: Benralizumab
Source: FDA, National Drug Code (US) Revision Year: 2024
1. Indications and Usage
FASENRA is indicated for the add-on maintenance treatment of patients aged 6 years and older with severe asthma, and with an eosinophilic phenotype [see Use in Specific Populations (8.4), Clinical Studies (14)].
Limitations of Use:
- FASENRA is not indicated for treatment of other eosinophilic conditions.
- FASENRA is not indicated for the relief of acute bronchospasm or status asthmaticus.
2. Dosage and Administration
2.1 Recommended Dosage
Adult and Adolescent Patients 12 Years of Age and Older
The recommended dosage of FASENRA is 30 mg (one injection) administered subcutaneously every 4 weeks for the first 3 doses, and then every 8 weeks thereafter.
Pediatric Patients 6 to 11 Years of Age
The recommended dosage of FASENRA for pediatric patients 6 to 11 years of age is based on body weight as provided in Table 1.
Table 1. Recommended Dosage of FASENRA in Pediatric Patients 6 to 11 Years of Age:
| Body weight | Recommended Dosage |
|---|---|
| Less than 35 kg | 10 mg (one injection) administered subcutaneously every 4 weeks for the first 3 doses, and then every 8 weeks thereafter. |
| 35 kg or more | 30 mg (one injection) administered subcutaneously every 4 weeks for the first 3 doses, and then every 8 weeks thereafter. |
2.2 General Administration Instructions
FASENRA is for subcutaneous use only.
FASENRA is intended for use under the guidance of a healthcare provider. In line with clinical practice, monitoring of patients after administration of biologic agents is recommended [see Warnings and Precautions (5.1)].
Administer FASENRA into the thigh or abdomen. The upper arm can also be used if a healthcare provider or caregiver administers the injection. Prior to administration, warm FASENRA by leaving carton at room temperature for about 30 minutes. Visually inspect FASENRA for particulate matter and discoloration prior to administration. FASENRA is clear to opalescent, colorless to slightly yellow, and may contain a few translucent or white to off-white particles. Do not use FASENRA if the liquid is cloudy, discolored, or if it contains large particles or foreign particulate matter.
Prefilled Syringe:
The prefilled syringe is for administration by a healthcare provider.
Autoinjector (FASENRA PEN):
FASENRA PEN is intended for administration by patients/caregivers. Patients/caregivers may inject after proper training in subcutaneous injection technique, and after the healthcare provider determines it is appropriate.
In patients aged 6 to 11 years weighing 35 kg or more, FASENRA PEN should only be administered by a caregiver or healthcare provider.
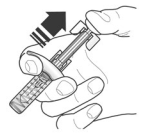
2.3 Instructions for Administration of FASENRA Prefilled Syringe (Healthcare Providers)
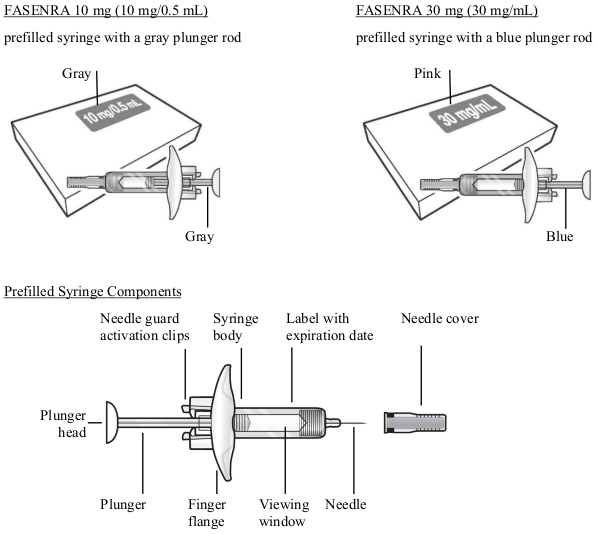
To prepare FASENRA prefilled syringe for subcutaneous administration, please carefully read and adhere to these instructions for use. FASENRA is available in a 10 mg and a 30 mg prefilled syringe. Check the labels on the FASENRA carton and prefilled syringe to ensure the correct 10 mg or 30 mg product is being used (Figure 1). Refer to Figure 1 to identify the prefilled syringe components for use in the administration steps.
Figure 1. FASENRA Prefilled Syringes:
Do not touch the needle guard activation clips to prevent premature activation of the needle safety guard.
1. Grasp the syringe body, not the plunger, to remove prefilled syringe from the tray. Check the expiration date on the syringe. The syringe may contain small air bubbles; this is normal. Do not expel the air bubbles prior to administration.
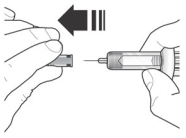
2. Do not remove needle cover until ready to inject. Hold the syringe body and remove the needle cover by pulling straight off. Do not hold the plunger or plunger head while removing the needle cover or the plunger may move. If the prefilled syringe is damaged or contaminated (for example, dropped without needle cover in place), discard and use a new prefilled syringe.
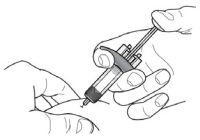
3. Gently pinch the skin and insert the needle at the recommended injection site (i.e., upper arm, thigh, or abdomen).
4. Inject all of the medication by pushing in the plunger all the way until the plunger head is completely between the needle guard activation clips. This is necessary to activate the needle guard.
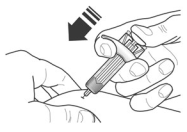
5. After injection, maintain pressure on the plunger head and remove the needle from the skin. Release pressure on the plunger head to allow the needle guard to cover the needle. Do not re-cap the prefilled syringe.
6. Discard the used syringe into a sharps container.
2.4 Instructions for Administration of FASENRA PEN
Refer to the FASENRA PEN ‘Instructions for Use’ for more detailed instructions on the preparation and administration of FASENRA PEN [see Instructions for Use]. A patient may self-inject or the patient’s caregiver may administer FASENRA PEN subcutaneously after the healthcare provider determines it is appropriate. In patients aged 6 to 11 years weighing 35 kg or more, FASENRA PEN should only be administered by a caregiver or healthcare provider.
10. Overdosage
Doses up to 200 mg were administered subcutaneously in clinical trials to patients with eosinophilic disease without evidence of dose-related toxicities.
There is no specific treatment for an overdose with benralizumab. If overdose occurs, the patient should be treated supportively with appropriate monitoring as necessary.
16.2. Storage and Handling
Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
If needed, the prefilled syringe and autoinjector may be stored at room temperature up to 77°F (25°C) for a maximum of 14 days in the original carton to protect from light. Once removed from the refrigerator and brought to room temperature (up to 77°F [25°C]), the prefilled syringe and autoinjector must be used within 14 days or discarded.
Do not freeze. Do not shake. Do not expose to heat.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.