HERCESSI Solution for injection Ref.[109764] Active ingredients: Trastuzumab
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
The HER2 (or c-erbB2) proto-oncogene encodes a transmembrane receptor protein of 185 kDa, which is structurally related to the epidermal growth factor receptor. Trastuzumab products have been shown, in both in vitro assays and in animals, to inhibit the proliferation of human tumor cells that overexpress HER2.
Trastuzumab products are mediators of antibody-dependent cellular cytotoxicity (ADCC). In vitro, trastuzumab product-mediated ADCC has been shown to be preferentially exerted on HER2 overexpressing cancer cells compared with cancer cells that do not overexpress HER2.
12.2. Pharmacodynamics
Cardiac Electrophysiology
The effects of trastuzumab on electrocardiographic (ECG) endpoints, including QTc interval duration, were evaluated in patients with HER2 positive solid tumors. Trastuzumab had no clinically relevant effect on the QTc interval duration and there was no apparent relationship between serum trastuzumab concentrations and change in QTcF interval duration in patients with HER2 positive solid tumors.
12.3. Pharmacokinetics
The pharmacokinetics of trastuzumab was evaluated in a pooled population pharmacokinetic (PK) model analysis of 1,582 subjects with primarily breast cancer and metastatic gastric cancer (MGC) receiving intravenous trastuzumab. Total trastuzumab clearance increases with decreasing concentrations due to parallel linear and non-linear elimination pathways.
Although the average trastuzumab exposure was higher following the first cycle in breast cancer patients receiving the three-weekly schedule compared to the weekly schedule of trastuzumab, the average steadystate exposure was essentially the same at both dosages. The average trastuzumab exposure following the first cycle and at steady state as well as the time to steady state was higher in breast cancer patients compared to MGC patients at the same dosage; however, the reason for this exposure difference is unknown. Additional predicted trastuzumab exposure and PK parameters following the first trastuzumab cycle and at steady state exposure are described in Tables 7 and 8, respectively.
Population PK based simulations indicate that following discontinuation of trastuzumab, concentrations in at least 95% of breast cancer and MGC patients will decrease to approximately 3% of the population predicted steady-state trough serum concentration (approximately 97% washout) by 7 months [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].
Table 7. Population Predicted Cycle 1 PK Exposures (Median with 5th – 95th Percentiles) in Breast Cancer and MGC Patients:
| Schedule | Primary tumor type | N | Cmin (µg/mL) | Cmax (µg/mL) | AUC0-21days (µg.day/mL) |
|---|---|---|---|---|---|
| 8 mg/kg + 6 mg/kg q3w | Breast cancer | 1195 | 29.4 (5.8 – 59.5) | 178 (117 – 291) | 1373 (736 – 2245) |
| MGC | 274 | 23.1 (6.1 – 50.3) | 132 (84.2 – 225) | 1109 (588 – 1938) | |
| 4 mg/kg + 2 mg/kg qw | Breast cancer | 1195 | 37.7 (12.3 – 70.9) | 88.3 (58 – 144) | 1066 (586 – 1754) |
Table 8. Population Predicted Steady State PK Exposures (Median with 5th – 95th Percentiles) in Breast Cancer and MGC Patients:
| Schedule | Primary tumor type | N | Cmin,ssa (µg/mL) | Cmax,ssb (µg/mL) | AUCss, 0-21 days (µg.day/mL) | Time to steadystate (week) | Total CL range at steadystate (L/day) |
|---|---|---|---|---|---|---|---|
| 8 mg/kg + 6 mg/kg q3w | Breast cancer | 1195 | 47.4 (5 – 115) | 179 (107 – 309) | 1794 (673 – 3618) | 12 | 0.173 – 0.283 |
| MGC | 274 | 32.9 (6.1 – 88.9) | 131 (72.5 – 251) | 1338 (557 – 2875) | 9 | 0.189 – 0.337 | |
| 4 mg/kg + 2 mg/kg qw | Breast cancer | 1195 | 66.1 (14.9 – 142) | 109 (51.0 – 209) | 1765 (647 – 3578) | 12 | 0.201 – 0.244 |
a Steady-state trough serum concentration of trastuzumab
b Maximum steady-state serum concentration of trastuzumab
Specific Populations
Based on a population pharmacokinetic analysis, no clinically significant differences were observed in the pharmacokinetics of trastuzumab based on age (< 65 (n = 1294); ≥ 65 (n = 288)), race (Asian (n = 264); nonAsian (n = 1324)) and renal impairment (mild (creatinine clearance [CLcr] 60 to 90 mL/min) (n = 636) or moderate (CLcr 30 to 60 mL/min) (n = 133)). The pharmacokinetics of trastuzumab products in patients with severe renal impairment, end-stage renal disease with or without hemodialysis, or hepatic impairment is unknown.
Drug Interaction Studies
There have been no formal drug interaction studies performed with trastuzumab products in humans. Clinically significant interactions between trastuzumab and concomitant medications used in clinical trials have not been observed.
Paclitaxel and doxorubicin: Concentrations of paclitaxel and doxorubicin and their major metabolites (i.e., 6-α hydroxyl-paclitaxel [POH], and doxorubicinol [DOL], respectively) were not altered in the presence of trastuzumab when used as combination therapy in clinical trials. Trastuzumab concentrations were not altered as part of this combination therapy.
Docetaxel and carboplatin: When trastuzumab was administered in combination with docetaxel or carboplatin, neither the plasma concentrations of docetaxel or carboplatin nor the plasma concentrations of trastuzumab were altered.
Cisplatin and capecitabine: In a drug interaction substudy conducted in patients in Study 7, the pharmacokinetics of cisplatin, capecitabine and their metabolites were not altered when administered in combination with trastuzumab.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Trastuzumab products have not been tested for carcinogenic potential.
No evidence of mutagenic activity was observed when trastuzumab was tested in the standard Ames bacterial and human peripheral blood lymphocyte mutagenicity assays at concentrations of up to 5000 mcg/mL. In an in vivo micronucleus assay, no evidence of chromosomal damage to mouse bone marrow cells was observed following bolus intravenous doses of up to 118 mg/kg of trastuzumab.
A fertility study was conducted in female cynomolgus monkeys at doses up to 25 times the weekly recommended human dose of 2 mg/kg of trastuzumab and has revealed no evidence of impaired fertility, as measured by menstrual cycle duration and female sex hormone levels.
14. Clinical Studies
14.1 Adjuvant Breast Cancer
The safety and efficacy of trastuzumab in women receiving adjuvant chemotherapy for HER2 overexpressing breast cancer were evaluated in an integrated analysis of two randomized, open-label, clinical trials (Studies 1 and 2) with a total of 4063 women at the protocol-specified final overall survival analysis, a third randomized, open-label, clinical trial (Study 3) with a total of 3386 women at definitive Disease-Free Survival analysis for one-year trastuzumab treatment versus observation, and a fourth randomized, openlabel clinical trial with a total of 3222 patients (Study 4).
Studies 1 and 2
In Studies 1 and 2, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH). HER2 testing was verified by a central laboratory prior to randomization (Study 2) or was required to be performed at a reference laboratory (Study 1). Patients with a history of active cardiac disease based on symptoms, abnormal electrocardiographic, radiologic, or left ventricular ejection fraction findings or uncontrolled hypertension (diastolic > 100 mm Hg or systolic > 200 mm Hg) were not eligible.
Patients were randomized (1:1) to receive doxorubicin and cyclophosphamide followed by paclitaxel (AC → paclitaxel) alone or paclitaxel plus trastuzumab (AC → paclitaxel + trastuzumab). In both trials, patients received four 21-day cycles of doxorubicin 60 mg/m² and cyclophosphamide 600 mg/m² . Paclitaxel was administered either weekly (80 mg/m² ) or every 3 weeks (175 mg/m² ) for a total of 12 weeks in Study 1; paclitaxel was administered only by the weekly schedule in Study 2. Trastuzumab was administered at 4 mg/kg on the day of initiation of paclitaxel and then at a dose of 2 mg/kg weekly for a total of 52 weeks.
Trastuzumab treatment was permanently discontinued in patients who developed congestive heart failure, or persistent/recurrent LVEF decline [see Dosage and Administration (2.3)]. Radiation therapy, if administered, was initiated after the completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. The primary endpoint of the combined efficacy analysis was Disease-Free Survival (DFS), defined as the time from randomization to recurrence, occurrence of contralateral breast cancer, other second primary cancer, or death. The secondary endpoint was overall survival (OS).
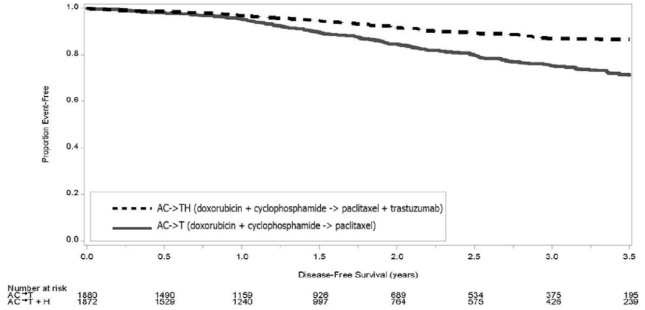
A total of 3752 patients were included in the joint efficacy analysis of the primary endpoint of DFS following a median follow-up of 2.0 years in the AC → paclitaxel + trastuzumab arm. The pre-planned final OS analysis from the joint analysis included 4063 patients and was performed when 707 deaths had occurred after a median follow-up of 8.3 years in the AC → paclitaxel + trastuzumab arm. The data from both arms in Study 1 and two of the three study arms in Study 2 were pooled for efficacy analyses. The patients included in the primary DFS analysis had a median age of 49 years (range, 22−80 years; 6% > 65 years), 84% were white, 7% black, 4% Hispanic, and 4% Asian/Pacific Islander. Disease characteristics included 90% infiltrating ductal histology, 38% T1, 91% nodal involvement, 27% intermediate and 66% high grade pathology, and 53% ER+ and/or PR+ tumors. Similar demographic and baseline characteristics were reported for the efficacy evaluable population, after 8.3 years of median follow-up in the AC → paclitaxel + trastuzumab arm.
Study 3
In Study 3, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH) as determined at a central laboratory. Patients with node-negative disease were required to have ≥ T1c primary tumor. Patients with a history of congestive heart failure or LVEF < 55%, uncontrolled arrhythmias, angina requiring medication, clinically significant valvular heart disease, evidence of transmural infarction on ECG, poorly controlled hypertension (systolic > 180 mm Hg or diastolic > 100 mm Hg) were not eligible.
Study 3 was designed to compare one and two years of three-weekly trastuzumab treatment versus observation in patients with HER2 positive EBC following surgery, established chemotherapy and radiotherapy (if applicable). Patients were randomized (1:1:1) upon completion of definitive surgery, and at least four cycles of chemotherapy to receive no additional treatment, or one year of trastuzumab treatment or two years of trastuzumab treatment. Patients undergoing a lumpectomy had also completed standard radiotherapy. Patients with ER+ and/or PgR+ disease received systemic adjuvant hormonal therapy at investigator discretion. Trastuzumab was administered with an initial dose of 8 mg/kg followed by subsequent doses of 6 mg/kg once every three weeks. The main outcome measure was Disease-Free Survival (DFS), defined as in Studies 1 and 2.
A protocol specified interim efficacy analysis comparing one-year trastuzumab treatment to observation was performed at a median follow-up duration of 12.6 months in the trastuzumab arm and formed the basis for the definitive DFS results from this study. Among the 3386 patients randomized to the observation (n = 1693) and trastuzumab one-year (n = 1693) treatment arms, the median age was 49 years (range 21−80), 83% were Caucasian, and 13% were Asian. Disease characteristics: 94% infiltrating ductal carcinoma, 50% ER+ and/or PgR+, 57% node positive, 32% node negative, and in 11% of patients, nodal status was not assessable due to prior neo-adjuvant chemotherapy. Ninety-six percent (1055/1098) of patients with nodenegative disease had high-risk features: among the 1098 patients with node-negative disease, 49% (543) were ER− and PgR−, and 47% (512) were ER and/or PgR+ and had at least one of the following high-risk features: pathological tumor size greater than 2 cm, Grade 2−3, or age < 35 years. Prior to randomization, 94% of patients had received anthracycline-based chemotherapy regimens.
After the definitive DFS results comparing observation to one-year trastuzumab treatment were disclosed, a prospectively planned analysis that included comparison of one year versus two years of trastuzumab treatment at a median follow-up duration of 8 years was performed. Based on this analysis, extending trastuzumab treatment for a duration of two years did not show additional benefit over treatment for one year [Hazard Ratios of two-years trastuzumab versus one-year trastuzumab treatment in the intent to treat (ITT) population for Disease-Free Survival (DFS) = 0.99 (95% CI: 0.87, 1.13), p-value = 0.90 and Overall Survival (OS) = 0.98 (0.83, 1.15); p-value = 0.78].
Study 4
In Study 4, breast tumor specimens were required to show HER2 gene amplification (FISH+ only) as determined at a central laboratory. Patients were required to have either node-positive disease, or nodenegative disease with at least one of the following high-risk features: ER/PR-negative, tumor size > 2 cm, age < 35 years, or histologic and/or nuclear Grade 2 or 3. Patients with a history of CHF, myocardial infarction, Grade 3 or 4 cardiac arrhythmia, angina requiring medication, clinically significant valvular heart disease, poorly controlled hypertension (diastolic > 100 mm Hg), any T4 or N2, or known N3 or M1 breast cancer were not eligible.
Patients were randomized (1:1:1) to receive doxorubicin and cyclophosphamide followed by docetaxel (ACT), doxorubicin and cyclophosphamide followed by docetaxel plus trastuzumab (AC-TH), or docetaxel and carboplatin plus trastuzumab (TCH). In both the AC-T and AC-TH arms, doxorubicin 60 mg/m² and cyclophosphamide 600 mg/m² were administered every 3 weeks for four cycles; docetaxel 100 mg/m² was administered every 3 weeks for four cycles. In the TCH arm, docetaxel 75 mg/m² and carboplatin (at a target AUC of 6 mg/mL/min as a 30- to 60-minute infusion) were administered every 3 weeks for six cycles. Trastuzumab was administered weekly (initial dose of 4 mg/kg followed by weekly dose of 2 mg/kg) concurrently with either T or TC, and then every 3 weeks (6 mg/kg) as monotherapy for a total of 52 weeks. Radiation therapy, if administered, was initiated after completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. Disease-Free Survival (DFS) was the main outcome measure.
Among the 3222 patients randomized, the median age was 49 (range 22 to 74 years; 6% ≥ 65 years). Disease characteristics included 54% ER+ and/or PR+ and 71% node positive. Prior to randomization, all patients underwent primary surgery for breast cancer.
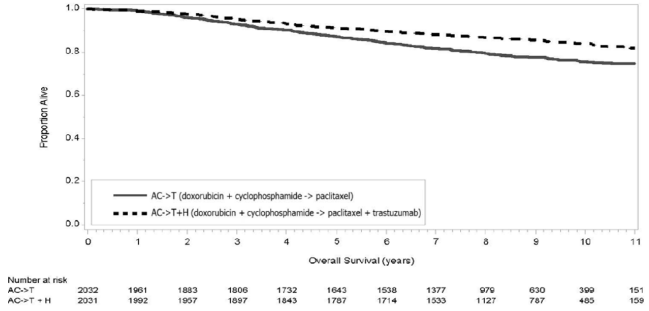
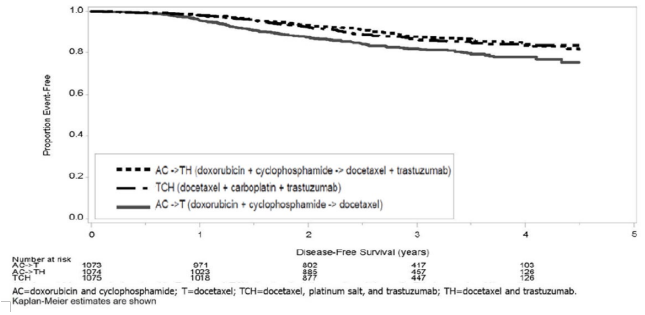
The results for DFS for the integrated analysis of Studies 1 and 2, Study 3, and Study 4 and OS results for the integrated analysis of Studies 1 and 2, and Study 3 are presented in Table 9. For Studies 1 and 2, the duration of DFS following a median follow-up of 2.0 years in the AC→TH arm is presented in Figure 4, and the duration of OS after a median follow-up of 8.3 years in the AC→TH arm is presented in Figure 5. The duration of DFS for Study 4 is presented in Figure 6. Across all four studies, at the time of definitive DFS analysis, there were insufficient numbers of patients within each of the following subgroups to determine if the treatment effect was different from that of the overall patient population: patients with low tumor grade, patients within specific ethnic/racial subgroups (Black, Hispanic, Asian/Pacific Islander patients), and patients >65 years of age. For Studies 1 and 2, the OS hazard ratio was 0.64 (95% CI: 0.55, 0.74). At 8.3 years of median follow-up [AC→TH], the survival rate was estimated to be 86.9% in the AC→TH arm and 79.4% in the AC→T arm. The final OS analysis results from Studies 1 and 2 indicate that OS benefit by age, hormone receptor status, number of positive lymph nodes, tumor size and grade, and surgery/radiation therapy was consistent with the treatment effect in the overall population. In patients ≤ 50 years of age (n = 2197), the OS hazard ratio was 0.65 (95% CI: 0.52, 0.81) and in patients > 50 years of age (n = 1866), the OS hazard ratio was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-positive disease (ER-positive and/or PR-positive) (n = 2223), the hazard ratio for OS was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-negative disease (ER-negative and PR-negative) (n = 1830), the hazard ratio for OS was 0.64 (95% CI: 0.52, 0.80). In the subgroup of patients with tumor size ≤ 2 cm (n = 1604), the hazard ratio for OS was 0.52 (95% CI: 0.39, 0.71). In the subgroup of patients with tumor size > 2 cm (n = 2448), the hazard ratio for OS was 0.67 (95% CI: 0.56, 0.80).
Table 9. Efficacy Results from Adjuvant Treatment of Breast Cancer (Studies 1 + 2, Study 3, and Study 4):
| DFS events | DFS Hazard ratio (95% CI) p-value | Deaths (OS events) | OS Hazard ratio p-value | |
|---|---|---|---|---|
| Studies 1 + 2a | ||||
| AC → TH (n = 1872)b (n = 2031)c | 133b | 0.48b,d (0.39, 0.59) p < 0.0001e | 289c | 0.64c,d (0.55, 0.74) p < 0.0001e |
| AC → T (n = 1880)b (n = 2032)c | 261b | 418c | ||
| Study 3f | ||||
| Chemo → Trastuzumab (n = 1693) | 127 | 0.54 (0.44, 0.67) p < 0.0001g | 31 | 0.75 p = NSh |
| Chemo → Observation (n = 1693) | 219 | 40 | ||
| Study 4i | ||||
| TCH (n = 1075) | 134 | 0.67 (0.54 – 0.84) p = 0.0006e,j | 56 | |
| AC → TH (n = 1074) | 121 | 0.60 (0.48 – 0.76) p < 0.0001e,i | 49 | |
| AC → T (n = 1073) | 180 | 80 | ||
CI = confidence interval.
a Studies 1 and 2 regimens: doxorubicin and cyclophosphamide followed by paclitaxel (AC→T) or paclitaxel plus trastuzumab (AC→TH).
b Efficacy evaluable population, for the primary DFS analysis, following a median follow-up of 2.0 years in the AC→TH arm.
c Efficacy evaluable population, for the final OS analysis, following 707 deaths (8.3 years of median followup in the AC→TH arm).
d Hazard ratio estimated by Cox regression stratified by clinical trial, intended paclitaxel schedule, number of positive nodes, and hormone receptor status.
e stratified log-rank test.
f At definitive DFS analysis with median duration of follow-up of 12.6 months in the one- year trastuzumab treatment arm.
g log-rank test. h NS = non-significant. i Study 4 regimens: doxorubicin and cyclophosphamide followed by docetaxel (AC→T) or docetaxel plus trastuzumab (AC→TH); docetaxel and carboplatin plus trastuzumab (TCH).
j A two-sided alpha level of 0.025 for each comparison.
Figure 4. Duration of Disease-Free Survival in Patients with Adjuvant Treatment of Breast Cancer (Studies 1 and 2):
Figure 5. Duration of Overall Survival in Patients with Adjuvant Treatment of Breast Cancer (Studies 1 and 2):
Figure 6. Duration of Disease-Free Survival in Patients with Adjuvant Treatment of Breast Cancer (Study 4):
Exploratory analyses of DFS as a function of HER2 overexpression or gene amplification were conducted for patients in Studies 2 and 3, where central laboratory testing data were available. The results are shown in Table 10. The number of events in Study 2 was small with the exception of the IHC 3+/FISH+ subgroup, which constituted 81% of those with data. Definitive conclusions cannot be drawn regarding efficacy within other subgroups due to the small number of events. The number of events in Study 3 was adequate to demonstrate significant effects on DFS in the IHC 3+/FISH unknown and the FISH+/IHC unknown subgroups.
Table 10. Treatment Outcomes in Studies 2 and 3 as a Function of HER2 Overexpression or Amplification:
| Study 2 | Study 3c | |||
|---|---|---|---|---|
| HER2 Assay Resulta | Number of Patients | Hazard Ratio DFS (95% CI) | Number of Patients | Hazard Ratio DFS (95% CI) |
| IHC 3+ | ||||
| FISH (+) | 1170 | 0.42 (0.27, 0.64) | 91 | 0.56 (0.13, 2.50) |
| FISH (−) | 51 | 0.71 (0.04, 11.79) | 8 | − |
| FISH Unknown | 51 | 0.69 (0.09, 5.14) | 2258 | 0.53 (0.41, 0.69) |
| IHC < 3+ / FISH (+) | 174 | 1.01 (0.18, 5.65) | 299b | 0.53 (0.20, 1.42) |
| IHC unknown / FISH (+) | − | − | 724 | 0.59 (0.38, 0.93) |
a IHC by HercepTest, FISH by PathVysion (HER2/CEP17 ratio ≥ 2.0) as performed at a central laboratory.
b All cases in this category in Study 3 were IHC 2+.
c Median follow-up duration of 12.6 months in the one-year trastuzumab treatment arm.
14.2 Metastatic Breast Cancer
The safety and efficacy of trastuzumab in treatment of women with metastatic breast cancer were studied in a randomized, controlled clinical trial in combination with chemotherapy (Study 5, n = 469 patients) and an open-label, single agent clinical trial (Study 6, n = 222 patients). Both trials studied patients with metastatic breast cancer whose tumors overexpress the HER2 protein. Patients were eligible if they had 2 or 3 levels of overexpression (based on a 0 to 3 scale) by immunohistochemical assessment of tumor tissue performed by a central testing lab.
Previously Untreated Metastatic Breast Cancer (Study 5)
Study 5 was a multicenter, randomized, open-label clinical trial conducted in 469 women with metastatic breast cancer who had not been previously treated with chemotherapy for metastatic disease. Tumor specimens were tested by IHC (Clinical Trial Assay, CTA) and scored as 0, 1+, 2+,or 3+, with 3+ indicating the strongest positivity. Only patients with 2+ or 3+ positive tumors were eligible (about 33% of those screened). Patients were randomized to receive chemotherapy alone or in combination with trastuzumab given intravenously as a 4 mg/kg loading dose followed by weekly doses of trastuzumab at 2 mg/kg. For those who had received prior anthracycline therapy in the adjuvant setting, chemotherapy consisted of paclitaxel (175 mg/m² over 3 hours every 21 days for at least six cycles); for all other patients, chemotherapy consisted of anthracycline plus cyclophosphamide (AC: doxorubicin 60 mg/m² or epirubicin 75 mg/m² plus 600 mg/m² cyclophosphamide every 21 days for six cycles). Sixty-five percent of patients randomized to receive chemotherapy alone in this study received trastuzumab at the time of disease progression aspart of a separate extension study.
Based upon the determination by an independent response evaluation committee, the patients randomized to trastuzumab and chemotherapy experienced a significantly longer median time to disease progression, a higher overall response rate (ORR), and a longer median duration of response as compared with patients randomized to chemotherapy alone. Patients randomized to trastuzumab and chemotherapy also had a longer median survival (see Table 11). These treatment effects were observed both in patients who received trastuzumab plus paclitaxel and in those who received trastuzumab plus AC; however the magnitude of the effects was greater in the paclitaxel subgroup.
Table 11. Study 5: Efficacy Results in First-Line Treatment for Metastatic Breast Cancer:
| Combined Results | Paclitaxel Subgroup | AC Subgroup | ||||
|---|---|---|---|---|---|---|
| Trastuzumab + All Chemotherapy (n = 235) | All Chemotherapy (n = 234) | Trastuzumab + Paclitaxel (n = 92) | Paclitaxel (n = 96) Trastuzumab + ACa (n = 143) | AC (n = 138) | ||
| Primary Endpoint | ||||||
| Median TTP (mos)b,c | 7.2 | 4.5 | 6.7 | 2.5 | 7.6 | 5.7 |
| 95% CI | 7, 8 | 4, 5 | 5, 10 | 2, 4 | 7, 9 | 5, 7 |
| p-valued | < 0.0001 | < 0.0001 | 0.002 | |||
| Secondary Endpoints | ||||||
| Overall Response Rateb | 45 | 29 | 38 | 15 | 50 | 38 |
| 95% CI | 39, 51 | 23, 35 | 28, 48 | 8, 22 | 42, 58 | 30, 46 |
| p-valuee | < 0.001 | < 0.001 | 0.10 | |||
| Median Resp Duration (mos)b,c | 8.3 | 5.8 | 8.3 | 4.3 | 8.4 | 6.4 |
| 25%, 75% Quartile | 6, 15 | 4, 8 | 5, 11 | 4, 7 | 6, 15 | 4, 8 |
| Med Survival (mos)c | 25.1 | 20.3 | 22.1 | 18.4 | 26.8 | 21.4 |
| 95% CI | 22, 30 | 17, 24 | 17, 29 | 13, 24 | 23, 33 | 18, 27 |
| p-valued | 0.05 | 0.17 | 0.16 | |||
a AC = Anthracycline (doxorubicin or epirubicin) and cyclophosphamide.
b Assessed by an independent Response Evaluation Committee.
c Kaplan-Meier Estimate.
d log-rank test. e χ2-test.
Data from Study 5 suggest that the beneficial treatment effects were largely limited to patients with the highest level of HER2 protein overexpression (3+) (see Table 12).
Table 12. Treatment Effects in Study 5 as a Function of HER2 Overexpression or Amplification:
| HER2 AssayResult | Number of Patients (N) | Relative Riskb for Time to Disease Progression (95% CI) | Relative Riskb for Mortality (95% CI) |
|---|---|---|---|
| CTA 2+ or 3+ | 469 | 0.49 (0.40, 0.61) | 0.80 (0.64, 1.00) |
| FISH (+)a | 325 | 0.44 (0.34, 0.57) | 0.70 (0.53, 0.91) |
| FISH (-)a | 126 | 0.62 (0.42, 0.94) | 1.06 (0.70, 1.63) |
| CTA 2+ | 120 | 0.76 (0.50, 1.15) | 1.26 (0.82, 1.94) |
| FISH (+) | 32 | 0.54 (0.21, 1.35) | 1.31 (0.53, 3.27) |
| FISH (-) | 83 | 0.77 (0.48, 1.25) | 1.11 (0.68, 1.82) |
| CTA 3+ | 349 | 0.42 (0.33, 0.54) | 0.70 (0.51, 0.90) |
| FISH (+) | 293 | 0.42 (0.32, 0.55) | 0.67 (0.51, 0.89) |
| FISH (-) | 43 | 0.43 (0.20, 0.94) | 0.88 (0.39, 1.98) |
a FISH testing results were available for 451 of the 469 patients enrolled on study.
b The relative risk represents the risk of progression or death in the trastuzumab plus chemotherapy arm versus the chemotherapy arm.
Previously Treated Metastatic Breast Cancer (Study 6)
Trastuzumab was studied as a single agent in a multicenter, open-label, single-arm clinical trial (Study 6) in patients with HER2 overexpressing metastatic breast cancer who had relapsed following one or two prior chemotherapy regimens for metastatic disease. Of 222 patients enrolled, 66% had received prior adjuvant chemotherapy, 68% had received two prior chemotherapy regimens for metastatic disease, and 25% had received prior myeloablative treatment with hematopoietic rescue. Patients were treated with a loading dose of 4 mg/kg IV followed by weekly doses of trastuzumab at 2 mg/kg IV.
The ORR (complete response + partial response), as determined by an independent Response Evaluation Committee, was 14%, with a 2% complete response rate and a 12% partial response rate. Complete responses were observed only in patients with disease limited to skin and lymph nodes. The overall response rate in patients whose tumors tested as CTA 3+ was 18% while in those that tested as CTA 2+, it was 6%.
14.3 Metastatic Gastric Cancer
The safety and efficacy of trastuzumab in combination with cisplatin and a fluoropyrimidine (capecitabine or 5-fluorouracil) were studied in patients previously untreated for metastatic gastric or gastroesophageal junction adenocarcinoma (Study 7). In this open-label, multi-center trial, 594 patients were randomized 1:1 to trastuzumab in combination with cisplatin and a fluoropyrimidine (FC+H) or chemotherapy alone (FC). Randomization was stratified by extent of disease (metastatic vs. locally advanced), primary site (gastric vs. gastroesophageal junction), tumor measurability (yes vs. no), ECOG performance status (0, 1 vs. 2), and fluoropyrimidine (capecitabine vs. 5-fluorouracil). All patients were either HER2 gene amplified (FISH+) or HER2 overexpressing (IHC 3+). Patients were also required to have adequate cardiac function (e.g., LVEF > 50%).
On the trastuzumab-containing arm, trastuzumab was administered as an IV infusion at an initial dose of 8 mg/kg followed by 6 mg/kg every 3 weeks until disease progression. On both study arms cisplatin was administered at a dose of 80 mg/m² Day 1 every 3 weeks for 6 cycles as a 2 hour IV infusion. On both study arms, capecitabine was administered at 1000 mg/m² dose orally twice daily (total daily dose 2000 mg/m² ) for 14 days of each 21 day cycle for 6 cycles. Alternatively, continuous intravenous infusion (CIV) 5-fluorouracil was administered at a dose of 800 mg/m² /day from Day 1 through Day 5 every three weeks for 6 cycles.
The median age of the study population was 60 years (range: 21−83); 76% were male; 53% were Asian, 38% Caucasian, 5% Hispanic, 5% other racial/ethnic groups; 91% had ECOG PS of 0 or 1; 82% had primary gastric cancer and 18% had primary gastroesophageal adenocarcinoma. Of these patients, 23% had undergone prior gastrectomy, 7% had received prior neoadjuvant and/or adjuvant therapy, and 2% had received prior radiotherapy.
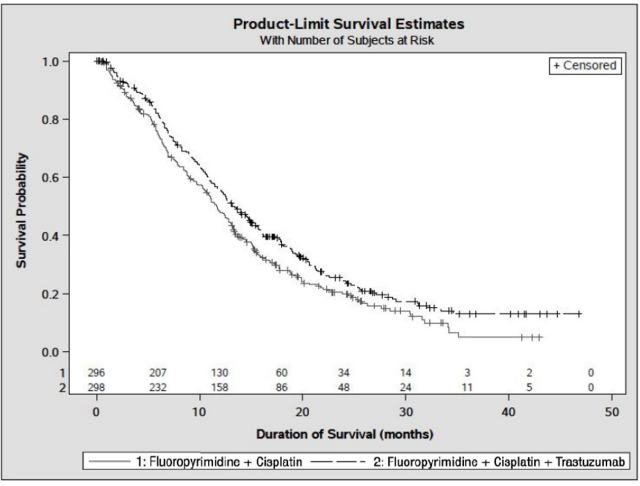
The main outcome measure of Study 7 was overall survival (OS), analyzed by the unstratified log-rank test. The final OS analysis based on 351 deaths was statistically significant (nominal significance level of 0.0193). An updated OS analysis was conducted at one year after the final analysis. The efficacy results of both the final and the updated analyses are summarized in Table 13 and Figure 7.
Table 13. Study 7: Overall Survival in ITT Population:
| FC Arm N = 296 | FC + H Arm N = 298 | ||
|---|---|---|---|
| Definitive (Second Interim) Overall Survival | |||
| No. Deaths (%) | 184 (62.2%) | 167 (56.0%) | |
| Median | 11.0 | 13.5 | |
| 95% CI (mos.) | (9.4, 12.5) | (11.7, 15.7) | |
| Hazard Ratio | 0.73 | ||
| 95% CI | (0.60, 0.91) | ||
| p-value*, two-sided | 0.0038 | ||
| Updated Overall Survival | |||
| No. Deaths (%) | 227 (76.7%) | 221 (74.2%) | |
| Median | 11.7 | 13.1 | |
| 95% CI (mos.) | (10.3, 13.0) | (11.9, 15.1) | |
| Hazard Ratio | 0.80 | ||
| 95% CI | (0.67, 0.97) | ||
* Comparing with the nominal significance level of 0.0193.
Figure 7. Updated Overall Survival in Patients with Metastatic Gastric Cancer (Study 7):
An exploratory analysis of OS in patients based on HER2 gene amplification (FISH) and protein overexpression (IHC) testing is summarized in Table 14.
Table 14. Exploratory Analyses by HER2 Status Using Updated Overall Survival Results:
| FC (N = 296)a | FC + H (N = 298)b | |
|---|---|---|
| FISH+ / IHC 0, 1+ subgroup (N=133) | ||
| No. Deaths / n (%) | 57/71 (80%) | 56/62 (90%) |
| Median OS Duration (mos.) | 8.8 | 8.3 |
| 95% CI (mos.) | (6.4, 11.7) | (6.2, 10.7) |
| Hazard ratio (95% CI) | 1.33 (0.92, 1.92) | |
| FISH+ / IHC2+ subgroup (N=160) | ||
| No. Deaths / n (%) | 65/80 (81%) | 64/80 (80%) |
| Median OS Duration (mos.) | 10.8 | 12.3 |
| 95% CI (mos.) | (6.8, 12.8) | (9.5, 15.7) |
| Hazard ratio (95% CI) | 0.78 (0.55, 1.10) | |
| FISH+ or FISH- / IHC3+c subgroup (N=294) | ||
| No. Deaths / n (%) | 104/143 (73%) | 96/151 (64%) |
| Median OS Duration (mos.) | 13.2 | 18.0 |
| 95% CI (mos.) | (11.5, 15.2) | (15.5, 21.2) |
| Hazard ratio (95% CI) | 0.66 (0.50, 0.87) | |
a Two patients on the FC arm who were FISH+ but IHC status unknown were excluded from the exploratory subgroup analyses.
b Five patients on the trastuzumab-containing arm who were FISH+, but IHC status unknown were excluded from the exploratory subgroup analyses.
c Includes 6 patients on chemotherapy arm, 10 patients on trastuzumab arm with FISH–, IHC3+ and 8 patients on chemotherapy arm, 8 patients on trastuzumab arm with FISH status unknown, IHC 3+.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.