INGREZZA Capsule Ref.[110006] Active ingredients: Valbenazine
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
The mechanism of action of valbenazine for the treatment of tardive dyskinesia and chorea in patients with Huntington’s disease is unclear, but is thought to be mediated through the reversible inhibition of vesicular monoamine transporter 2 (VMAT2), a transporter that regulates monoamine uptake from the cytoplasm to the synaptic vesicle for storage and release.
12.2. Pharmacodynamics
Valbenazine inhibits human VMAT2 (Ki ~150 nM) with no appreciable binding affinity for VMAT1 (Ki >10 µM). Valbenazine is converted to the active metabolite [+]-α-dihydrotetrabenazine ([ + ]-α-HTBZ).
[+]-α-HTBZ also binds with relatively high affinity to human VMAT2 (Ki ~3 nM).
Valbenazine and [+]-α-HTBZ have no appreciable binding affinity (Ki >5000 nM) for dopaminergic (including D2), serotonergic (including 5HT2B), adrenergic, histaminergic or muscarinic receptors.
Cardiac Electrophysiology
INGREZZA and INGREZZA SPRINKLE may cause an increase in the corrected QT interval in patients who are CYP2D6 poor metabolizers or who are taking a strong CYP2D6 or CYP3A4 inhibitor. An exposure-response analysis of clinical data from two healthy volunteer studies revealed increased QTc interval with higher plasma concentrations of the active metabolite. Based on this model, patients taking an INGREZZA 60 mg or 80 mg dose with increased exposure to the metabolite (e.g., being a CYP2D6 poor metabolizer) may have a mean (upper bound of double-sided 90% CI) QT prolongation of 9.6 (12.0) msec or 11.7 (14.7) msec, respectively as compared to otherwise healthy volunteers given INGREZZA, who had a respective mean (upper bound of double-sided 90% CI) QT prolongation of 5.3 (6.7) msec or 6.7 (8.4) msec [see Warnings and Precautions (5.4)].
12.3. Pharmacokinetics
Valbenazine and its active metabolite ([+]-α-HTBZ) demonstrate approximate proportional increases for the area under the plasma concentration versus time curve (AUC) and maximum plasma concentration (Cmax) after single oral doses from 40 mg to 300 mg (i.e., 50% to 375% of the recommended treatment dose).
Absorption
INGREZZA
Following oral administration of INGREZZA, the time to reach maximum valbenazine plasma concentration (tmax) ranges from 0.5 to 1.0 hours. Valbenazine reaches steady state plasma concentrations within 1 week. The absolute oral bioavailability of valbenazine is approximately 49%. [+]-α-HTBZ gradually forms and reaches Cmax 4 to 8 hours after administration of INGREZZA.
INGREZZA SPRINKLE
Following administration of 80 mg INGREZZA SPRINKLE orally as sprinkle on applesauce, the geometric mean peak plasma concentration (Cmax) of valbenazine was 512 ng/mL, area under the plasma concentration-time curve (AUCinf) was 5,600 ng*hr/mL and the median time to reach Cmax (Tmax) was 1.5 hours. For the [+]-α-HTBZ metabolite, the geometric mean Cmax was 23 ng/mL, AUCinf was 739 ng*hr/mL and the median Tmax was 6 hours.
Following oral administration of 80 mg INGREZZA, the geometric mean Cmax, AUCinf and median Tmax of valbenazine were 744 ng/mL, 6,419 ng*hr/mL and 0.5 hour, respectively. For the [+]-α-HTBZ metabolite, the geometric mean Cmax was 26 ng/mL, AUCinf was 859 ng*hr/mL and the median Tmax was 6 hours.
When 80 mg INGREZZA SPRINKLE capsules were swallowed whole with water, the geometric mean valbenazine Cmax, AUCinf and median Tmax were 685 ng/mL, 5,981 ng*hr/mL and 1 hour, respectively. For the [+]-α-HTBZ metabolite, the geometric mean Cmax was 23 ng/mL, AUCinf was 772 ng*hr/mL and the median Tmax was 6 hours.
Effect of Food
INGREZZA:
Ingestion of a high-fat meal decreases valbenazine Cmax by approximately 47% and AUC by approximately 13%. [+]-α-HTBZ Cmax and AUC are unaffected.
INGREZZA SPRINKLE:
Ingestion of a high-fat meal decreases valbenazine Cmax by approximately 15% and did not have an appreciable effect on AUC. [+]-α-HTBZ Cmax and AUC are unaffected.
Distribution
The plasma protein binding of valbenazine and [+]-α-HTBZ are greater than 99% and approximately 64%, respectively. The mean steady state volume of distribution of valbenazine is 92 L.
Nonclinical data in Long-Evans rats show that valbenazine can bind to melanin-containing structures of the eye such as the uveal tract. The relevance of this observation to clinical use of INGREZZA or INGREZZA SPRINKLE is unknown.
Elimination
Valbenazine has a mean total plasma systemic clearance value of 7.2 L/hr. Valbenazine and [+]-α-HTBZ have half-lives of 15 to 22 hours.
Metabolism
Valbenazine is extensively metabolized after oral administration by hydrolysis of the valine ester to form the active metabolite ([ + ]-α-HTBZ) and by oxidative metabolism, primarily by CYP3A4/5, to form mono-oxidized valbenazine and other minor metabolites. [+]-α-HTBZ appears to be further metabolized in part by CYP2D6.
Excretion
Following the administration of a single 50-mg oral dose of radiolabeled C-valbenazine (i.e., ~63% of the recommended treatment dose), approximately 60% and 30% of the administered radioactivity was recovered in the urine and feces, respectively. Less than 2% was excreted as unchanged valbenazine or [+]-α-HTBZ in either urine or feces.
Specific Populations
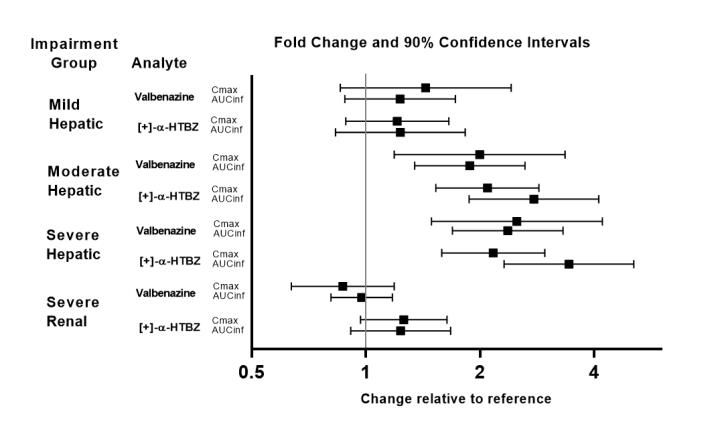
Exposures of valbenazine in patients with hepatic and severe renal impairment are summarized in Figure 1.
Figure 1. Effects of Hepatic and Severe Renal Impairment on Valbenazine Pharmacokinetics:
AUCinf=area under the plasma concentration versus time curve from 0 hours extrapolated to infinity
[ + ]-α-HTBZ=[+]-α-dihydrotetrabenazine (active metabolite)
Drug Interaction Studies
In vivo Drug Interactions
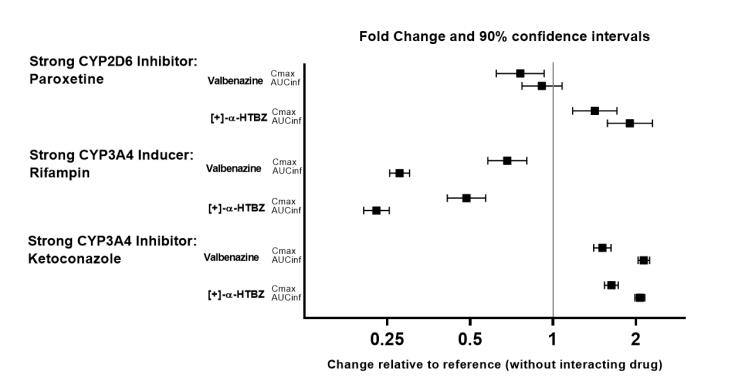
The effects of paroxetine, ketoconazole and rifampin on the exposure of valbenazine are summarized in Figure 2.
Figure 2. Effects of Strong CYP2D6 and CYP3A4 Inhibitors and CYP3A4 Inducers on Valbenazine Pharmacokinetics:
AUCinf=area under the plasma concentration versus time curve from 0 hours extrapolated to infinity
[ + ]-α-HTBZ=[+]-α-dihydrotetrabenazine (active metabolite)
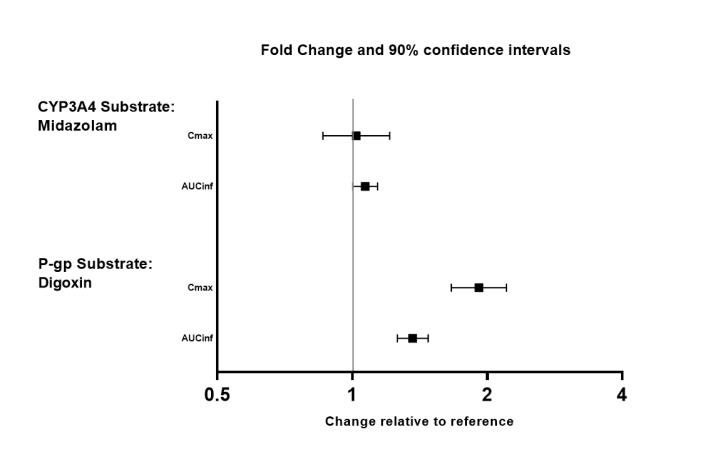
The effects of valbenazine on the exposure of other coadministered drugs are summarized in Figure 3.
Figure 3. Effects of Valbenazine on Pharmacokinetics of Other Drugs:
AUCinf=area under the plasma concentration versus time curve from 0 hours extrapolated to infinity
In vitro Drug Interactions
The results of in vitro studies suggest that valbenazine and [+]-α-HTBZ are unlikely to inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1 or CYP3A4/5, or induce CYP1A2, CYP2B6 or CYP3A4/5 at clinically relevant concentrations.
The results of in vitro studies suggest that valbenazine and [+]-α-HTBZ are unlikely to inhibit the transporters (BCRP, OAT1, OAT3, OCT2, OATP1B1, or OATP1B3) at clinically relevant concentrations.
12.5. Pharmacogenomics
CYP2D6 metabolizes the active metabolite of valbenazine ([+]-α-HTBZ). The gene encoding CYP2D6 has polymorphisms that impact protein function. CYP2D6 poor metabolizers are individuals with two non-functioning alleles, resulting in no enzyme activity.
Pharmacokinetic data from CYP2D6 poor metabolizers (n=25) treated with valbenazine demonstrate an approximate 2-fold higher AUCinf and a 1.8-fold higher Cmax of [+]-α-HTBZ compared to normal metabolizers. Dosage reduction is recommended in CYP2D6 poor metabolizers [see Dosage and Administration (2.4), Warnings and Precautions (5.4), and Use in Specific Populations (8.6)].
In a clinical study, AUC of [ + ]-α-HTBZ was 22% higher and Cmax was 9% lower in intermediate metabolizers (n=7) as compared to normal metabolizers (n=11), which is not considered clinically relevant. The effects of ultrarapid metabolizer status on the pharmacokinetics of [+]-α-HTBZ have not been studied.
Approximately 7% of White populations, 2% of Asian populations, and 2% of African-American populations are poor metabolizers.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Valbenazine did not increase tumors in rats treated orally for 91 weeks at 0.5, 1, and 2 mg/kg/day. These doses are <1 times (0.06, 0.1, and 0.24 times, respectively) the MRHD of 80 mg/day based on mg/m².
Valbenazine did not increase tumors in hemizygous Tg.rasH2 mice treated orally for 26 weeks at 10, 30 and 75 mg/kg/day, which are 0.6, 1.9 and 4.6 times the MRHD of 80 mg/day based on mg/m².
Mutagenesis
Valbenazine was not mutagenic in the in vitro bacterial reverse mutation test (Ames) or clastogenic in the in vitro mammalian chromosomal aberrations assay in human peripheral blood lymphocytes or in the in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
In a fertility study, rats were treated orally with valbenazine at 1, 3, and 10 mg/kg/day prior to mating and through mating, for a minimum of 10 weeks (males) or through Day 7 of gestation (females). These doses are 0.1, 0.4, and 1.2 times the MRHD of 80 mg/day based on mg/m², respectively. Valbenazine delayed mating in both sexes, which led to lower number of pregnancies and disrupted estrous cyclicity at the high dose, 1.2 times the MRHD of 80 mg/day based on mg/m². Valbenazine had no effects on sperm parameters (motility, count, density) or on uterine parameters (corpora lutea, number of implants, viable implants, pre-implantation loss, early resorptions and post-implantation loss) at any dose.
14. Clinical Studies
14.1 Tardive Dyskinesia
INGREZZA SPRINKLE
The effectiveness of INGREZZA SPRINKLE has been established from adequate and well-controlled studies of INGREZZA for the treatment of tardive dyskinesia. Presented below is a display of the efficacy results of the adequate and well-controlled studies of INGREZZA in patients with tardive dyskinesia.
INGREZZA
A randomized, double-blind, placebo-controlled trial of INGREZZA was conducted in patients with moderate to severe tardive dyskinesia as determined by clinical observation. Patients had underlying schizophrenia, schizoaffective disorder, or a mood disorder. Individuals at significant risk for suicidal or violent behavior and individuals with unstable psychiatric symptoms were excluded.
The Abnormal Involuntary Movement Scale (AIMS) was the primary efficacy measure for the assessment of tardive dyskinesia severity. The AIMS is a 12-item scale; items 1 to 7 assess the severity of involuntary movements across body regions and these items were used in this study. Each of the 7 items was scored on a 0 to 4 scale, rated as: 0=no dyskinesia; 1=low amplitude, present during some but not most of the exam; 2=low amplitude and present during most of the exam (or moderate amplitude and present during some of the exam); 3=moderate amplitude and present during most of exam; or 4=maximal amplitude and present during most of exam. The AIMS dyskinesia total score (sum of items 1 to 7) could thus range from 0 to 28, with a decrease in score indicating improvement. The AIMS was scored by central raters who interpreted the videos blinded to subject identification, treatment assignment, and visit number.
The primary efficacy endpoint was the mean change from baseline in the AIMS dyskinesia total score at the end of Week 6. The change from baseline for two fixed doses of INGREZZA (40 mg or 80 mg) was compared to placebo. At the end of Week 6, subjects initially assigned to placebo were re-randomized to receive INGREZZA 40 mg or 80 mg. Subjects originally randomized to INGREZZA continued INGREZZA at their randomized dose. Follow-up was continued through Week 48 on the assigned drug, followed by a 4-week period off-drug (subjects were not blind to withdrawal).
A total of 234 subjects were enrolled, with 29 (12%) discontinuing prior to completion of the placebo-controlled period. Mean age was 56 (range 26 to 84). Patients were 54% male and 46% female. Patients were 57% Caucasian, 38% African-American, and 5% other. Concurrent diagnoses included schizophrenia/schizoaffective disorder (66%) and mood disorder (34%). With respect to concurrent antipsychotic use, 70% of subjects were receiving atypical antipsychotics, 14% were receiving typical or combination antipsychotics, and 16% were not receiving antipsychotics.
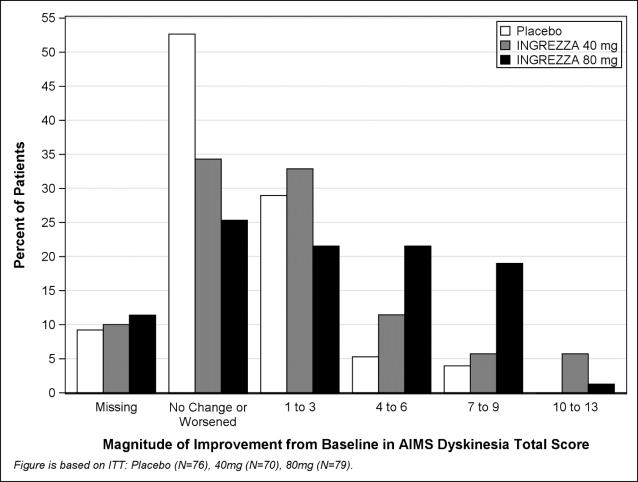
Results are presented in Table 4, with the distribution of responses shown in Figure 4. The change from baseline in the AIMS total dyskinesia score in the 80 mg INGREZZA group was statistically significantly different from the change in the placebo group. Subgroup analyses by gender, age, racial subgroup, underlying psychiatric diagnostic category, and concomitant antipsychotic medication did not suggest any clear evidence of differential responsiveness.
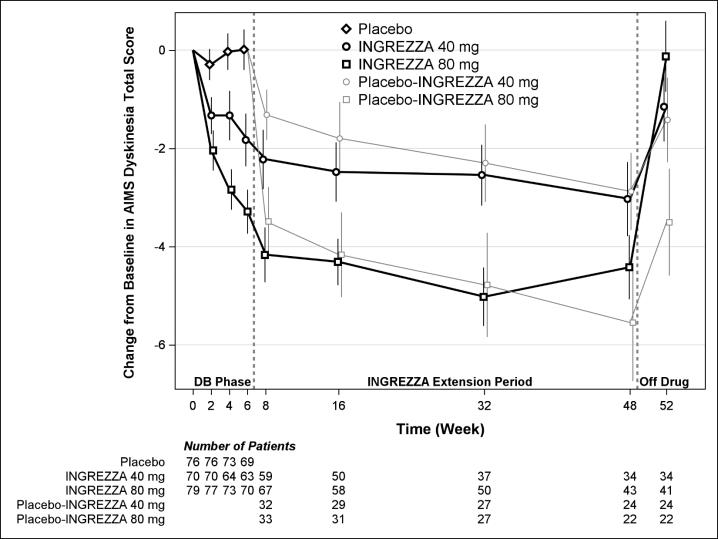
The mean changes in the AIMS dyskinesia total score by visit are shown in Figure 5. Among subjects remaining in the study at the end of the 48-week treatment (N=123 [52.6%]), following discontinuation of INGREZZA, the mean AIMS dyskinesia total score appeared to return toward baseline (there was no formal hypothesis testing for the change following discontinuation).
Table 4. Primary Efficacy Endpoint – Severity of Tardive Dyskinesia at Baseline and the End of Week 6:
| Endpoint | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SEM)** | Placebo- subtracted Difference (95% CI) |
|---|---|---|---|---|
| AIMS Dyskinesia Total Score | INGREZZA 40 mg | 9.8 (4.1) | -1.9 (0.4) | -1.8 (-3.0, -0.7) |
| INGREZZA 80 mg* | 10.4 (3.6) | -3.2 (0.4) | -3.1 (-4.2, -2.0) | |
| Placebo | 9.9 (4.3) | -0.1 (0.4) |
LS Mean = least-squares mean; SD = standard deviation; SEM = standard error of the mean; CI = 2-sided 95% confidence interval
* Dose that was statistically significantly different from placebo after adjusting for multiplicity.
** A negative change from baseline indicates improvement.
Figure 4. Percent of Patients with Specified Magnitude of AIMS Total Score Improvement at the End of Week 6:
ITT = Intent to Treat; This analysis set includes all randomized patients who had a baseline and at least one post-baseline AIMS dyskinesia total score value reported.
Figure 5. AIMS Dyskinesia Total Score Mean Change from Baseline – Entire Study Duration (Arithmetic Mean):
DB = Double-Blind; After Week 6, subjects initially receiving placebo were re-randomized to receive INGREZZA 40 mg or 80 mg until the end of Week 48. Error bars represent ±1 Standard Error of the Mean (SEM).
Efficacy of INGREZZA 60 mg
Based on modeling and simulation, the predicted mean change from baseline in the AIMS dyskinesia total score at Week 6 for INGREZZA 60 mg once daily in subjects with TD is -2.69 (95% CI: -3.30, -2.13), which is within the efficacy range for INGREZZA 40 mg and 80 mg once daily.
14.2 Chorea Associated with Huntington 's Disease
INGREZZA SPRINKLE
The effectiveness of INGREZZA SPRINKLE has been established from adequate and well-controlled studies of INGREZZA for the treatment of chorea associated with Huntington’s disease. Presented below is a display of the efficacy results of the adequate and well-controlled studies of INGREZZA in patients with chorea associated with Huntington’s disease.
INGREZZA
A randomized, double-blind, placebo-controlled study was conducted to evaluate the efficacy, safety, and tolerability of INGREZZA in patients with chorea associated with Huntington’s disease (NCT04102579). Treatment duration was 12 weeks followed by a 2-week period off drug. INGREZZA was started at 40 mg per day and the dose could be increased every 2 weeks in 20 mg increments up to a maximum dosage of 80 mg per day. The primary efficacy endpoint was the change from baseline to the end of the treatment period (average of Week 10 and Week 12) in the Total Maximal Chorea score of the Unified Huntington’s Disease Rating Scale (UHDRS). The Total Maximal Chorea score is rated from 0 to 4 (with 0 representing no chorea) for 7 different parts of the body, with a total score ranging from 0 to 28.
A total of 128 patients were randomized into the study, and 125 patients were included in the analysis of efficacy. In these patients, the mean age was 54 years (range 25 to 74 years), 46% were male and 96% were White. Greater than 80% of patients were taking the 80 mg daily dosage at the end of the 12-week treatment period.
Table 5 and Figure 6 summarize the effects of INGREZZA on chorea based on the Total Maximal Chorea score.
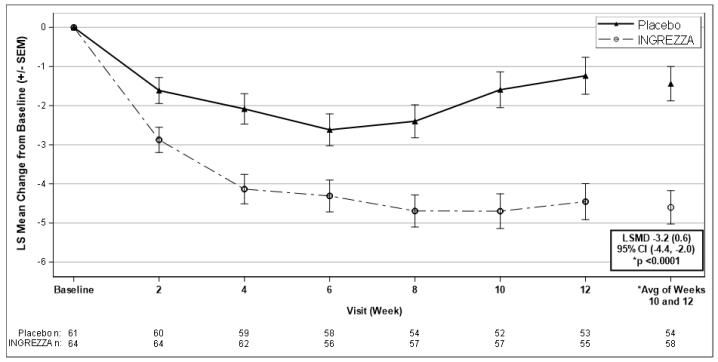
The mean change in Total Maximal Chorea scores for patients receiving INGREZZA improved by 4.6 units (LS mean) from baseline to the end of the treatment period (average of Week 10 and Week 12), compared to 1.4 units in the placebo group. The treatment effect of -3.2 units was statistically significant (p<0.0001) (Figure 6). At the Week 14 follow-up visit (2 weeks after discontinuation of the study medication), the Total Maximal Chorea scores of patients who had received INGREZZA returned to baseline.
Table 5. Primary Efficacy Endpoint – Mean Change from Baseline to End of Treatment in Total Maximal Chorea Score in Patients with Huntington’s Disease Treated with INGREZZA:
| Primary Endpoint | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SEM)b | Placebo- subtracted Difference (95% CI) |
|---|---|---|---|---|
| TMC Scorea | INGREZZA N=64 | 12.2 (2.3) | -4.6 (0.4) | -3.2 (-4.4, -2.0) p<0.0001 |
| Placebo N=61 | 12.1 (2.8) | -1.4 (0.4) |
a TMC, Total Maximal Chorea is a subscale of the Unified Huntington’s Disease Rating Scale (UHDRS)
b LS Mean = least-squares mean; SD = standard deviation; SEM=standard error of the mean; CI = 2-sided 95% confidence interval
Figure 6. Mean Change in Total Maximal Chorea Score Over Time:
LSMD = least-squares mean difference (SEM) [INGREZZA – Placebo]
CI = confidence interval
SEM = standard error of the mean
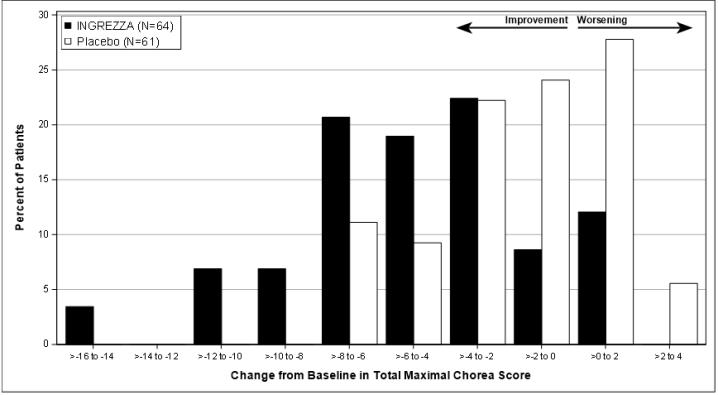
Figure 7. Distribution of the Change in Total Maximal Chorea Score (Average of Week 10 and 12):
Figure 7 shows the distribution of values for the change in Total Maximal Chorea Score. Negative values indicate a reduction in chorea and positive values indicate an increase in chorea.
In a clinician-rated global impression of change (CGI-C), clinicians rated 43% of patients treated with INGREZZA as “Much Improved” or “Very Much Improved” at the end of treatment, compared to 13% of patients who received placebo (p<0.001).
A patient-rated global impression of change (PGI-C) assessed how patients rated their overall chorea symptoms. Of the patients treated with INGREZZA, 53% rated their symptoms as “Much Improved” or “Very Much Improved” at the end of treatment, compared to 26% of patients who received placebo (p<0.01).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.