LETYBO Powder for solution Ref.[109317] Active ingredients: Botulinum toxin type A
Source: FDA, National Drug Code (US) Revision Year: 2024
1. Indications and Usage
LETYBO is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in adult patients.
2. Dosage and Administration
2.1 Important Administration Instructions
The potency Units of LETYBO (letibotulinumtoxinA-wlbg) for injection are specific to the preparation and assay utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of LETYBO cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay [see Warnings and Precautions (5.2) and Description (11)].
LETYBO should be administered no more frequently than every three months. Consideration of the cumulative dose is necessary when treating adult patients with LETYBO for glabellar lines if other botulinum toxin products are or have been used to treat other indications approved for those products.
The safe and effective use of LETYBO depends upon proper storage of the product, selection of the correct dose, and proper reconstitution and administration techniques.LETYBO
After reconstitution, only use each LETYBO vial for one injection session and for only one patient. Discard any remaining solution in vial immediately after administration.
Reconstitution instructions are provided specifically for the 50 Unit and the 100 Unit vials (Table 1)
2.2 Recommended Dosage
The total recommended dose is 20 Units per treatment session divided into five equal intramuscular injections of 4 Units each (two injections in each corrugator muscle and one injection in the procerus muscle).
2.3 Preparation and Dilution Technique
LETYBO is supplied in a single-dose 50 or 100-Unit vial. Prior to intramuscular injection, reconstitute each freeze-dried vial of LETYBO with the required amount of sterile, preservative-free 0.9% Sodium Chloride Injection, USP to achieve a reconstituted solution at a concentration of 4 Units/0.1 mL (see Table 1).
Table 1. Dilution Instructions for LETYBO Vials (50 and 100 Units):
| Vial | Amount of Diluent* Added | Resulting Dose Units per 0.1 mL |
|---|---|---|
| 50 Units | 1.25 mL | 4 Units |
| 100 Units | 2.5 mL | 4 Units |
* Preservative-free 0.9% Sodium Chloride Injection, USP
Slowly inject the diluent into the vial. Discard the vial if a vacuum does not pull the diluent into the vial. Dispose of any unused diluent. Gently mix LETYBO with 0.9% sodium chloride injection, USP by rotating the vial.
Reconstituted LETYBO is clear and colorless, and free of particulate matter. Inspect visually the reconstituted LETYBO for particulate matter and discoloration prior to administration. Do not use if the solution is cloudy or discolored or contains flakes or particles.
Administer LETYBO within 24 hours after reconstitution. During this time period, store unused reconstituted LETYBO in a refrigerator between 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze reconstituted LETYBO.
2.4 Administraton
The upper eyelid margin position should be carefully examined for separation or weakness of the levator palpebrae superioris muscle. Evaluate the range of upper eyelid excursion while manually immobilizing the frontalis to assess degree of levator function and frontalis compensation.
In order to reduce the complication of eyelid ptosis the following steps should be taken:
- Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
- Ensure the injected volume/dose is accurate and administer in a steady, controlled manner.
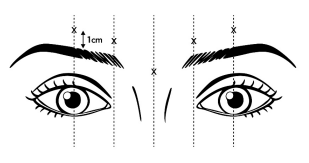
- Lateral corrugator injections should be placed at least 1 cm above the bony supraorbital ridge.
- Avoid injecting toxin closer than 1 centimeter above the central eyebrow.
Draw at least 0.5 mL of the properly reconstituted toxin into a sterile syringe and expel any air bubbles in the syringe barrel. Remove the needle used to reconstitute the product and attach a 30-31 gauge needle. Confirm the patency of the needle. Inject a dose of 0.1 mL (4 Units) intramuscularly into each of 5 sites, the inferomedial and superior middle of each corrugator and one in the mid-line of the procerus muscle for a total dose of 20 Units (see Figure 1).
Figure 1. LETYBO Sites (x) for Intramuscular Injection:
10. Overdosage
Excessive doses of LETYBO may be expected to produce neuromuscular weakness with a variety of symptoms. Respiratory support may be required where excessive doses cause paralysis of the respiratory muscles. Symptoms of overdose are likely not to be present immediately following injection. Should accidental injection or oral ingestion occur, or overdose be suspected, the person should be medically supervised for several weeks for signs and symptoms of systemic muscular weakness or paralysis.
In the event of overdose, antitoxin raised against botulinum toxin is available from the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. However, the antitoxin will not reverse any botulinum toxininduced effects already apparent by the time of antitoxin administration. In the event of suspected or actual cases of botulinum toxin poisoning, please contact your local or state Health Department to process a request for antitoxin through the CDC. If you do not receive a response within 30 minutes, please contact the CDC directly at 1-770-488-7100.
16.2. Storage and Handling
Unopened LETYBO vials should be stored in a refrigerator between 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
Do not freeze.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.