MULTAQ Film-coated tablet Ref.[6810] Active ingredients: Dronedarone
Source: European Medicines Agency (EU) Revision Year: 2018 Publisher: sanofi-aventis groupe, 54, rue La Boétie, F-75008, Paris, France
Pharmacodynamic properties
Pharmacotherapeutic group: cardiac therapy, antiarrhythmic class III
ATC code: C01BD07
Mechanism of action
In animals, dronedarone prevents atrial fibrillation or restores normal sinus rhythm depending on the model used. It also prevents ventricular tachycardia and ventricular fibrillation in several animal models. These effects most likely result from its electrophysiological properties belonging to all four Vaughan-Williams classes. Dronedarone is a multichannel blocker inhibiting the potassium currents (including IK(Ach), IKur, IKr, IKs) and thus prolonging cardiac action potential and refractory periods (Class III). It also inhibits the sodium currents (Class Ib) and the calcium currents (Class IV). It non-competitively antagonises adrenergic activities (Class II).
Pharmacodynamic properties
In animal models, dronedarone reduces the heart rate. It prolongs Wenckebach cycle length and AH-, PQ-, QT- intervals; with no marked effect or weak increase on QTc-intervals, and with no change in HV- and QRS- intervals. It increases effective refractory periods (ERP) of the atrium, atrio-ventricular node, and ventricular ERP was slightly prolonged with a minimal degree of reverse frequency dependency.
Dronedarone decreases arterial blood pressure and myocardial contractility (dP/dt max) with no change in left ventricular ejection fraction and reduces myocardial oxygen consumption.
Dronedarone has vasodilatory properties, in coronary arteries (related to the activation of the nitric oxide pathway) and in peripheral arteries.
Dronedarone displays indirect antiadrenergic effects and partial antagonism to adrenergic stimulation. It reduces alpha-adrenergic blood pressure response to epinephrine and beta1 and beta2 responses to isoproterenol.
Clinical efficacy and safety
Reduction of risk of AF-related hospitalisation
The efficacy of dronedarone in the reduction of risk of AF-related hospitalisation was demonstrated in patients with AF or a history of AF and additional risk factors in the ATHENA multicenter, multinational, double blind, and randomised placebo-controlled study. Patients were to have at least one risk factor (including age, hypertension, diabetes, prior cerebrovascular accident, left atrium diameter ≥50 mm or LVEF <0.40) together with AF/AFL and sinus rhythm both documented within the last 6 months. Patients who received amiodarone within 4 weeks prior to randomisation were not included. Patients could be in AF/AFL or in sinus rhythm after spontaneous conversion or following any procedures.
Four thousand six hundred and twenty eight (4,628) patients were randomised and treated for up to 30 months maximum (median follow-up: 22 months) with either dronedarone 400 mg twice daily (2,301 patients) or placebo (2,327 patients), in addition to conventional therapy including beta-blockers (71%), ACE inhibitors or AIIRAs (69%) digitalis (14%), calcium antagonists (14%), statins (39%), oral anticoagulants (60%), chronic antiplatelet therapy (6%) and/or diuretics (54%).
The primary endpoint of the study was the time to first hospitalisation for cardiovascular reasons or death from any cause.
Patients ranged in age from 23 to 97 years and 42% were over 75 years old. Forty seven percent (47%) of patients were female and a majority was Caucasian (89%).
The majority had hypertension (86%) and structural heart disease (60%) (including coronary artery disease: 30%; congestive heart failure (CHF): 30%; LVEF<45%: 12%). Twenty five percent (25%) had AF at baseline.
Dronedarone reduced the incidence of cardiovascular hospitalisation or death from any cause by 24.2% when compared to placebo (p<0.0001).
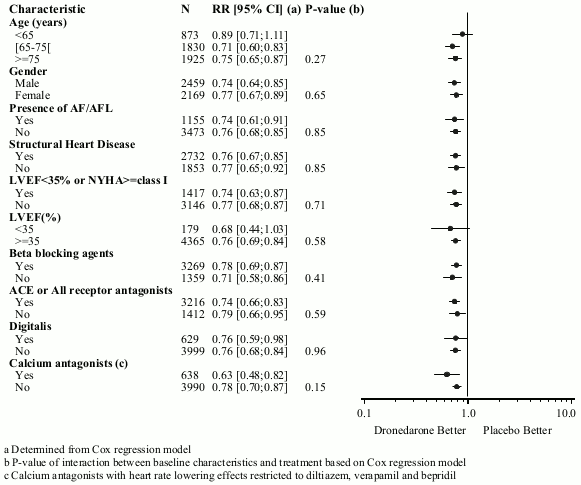
The reduction in cardiovascular hospitalisation or death from any cause was consistent in all subgroups, irrespective of baseline characteristics or medicinal products (ACE inhibitors or AIIRAs; beta-blockers, digitalis, statins, calcium antagonists, diuretics) (see figure 1).
Figure 1. Relative risk (dronedarone 400 mg twice daily versus placebo) estimates with 95% confidence intervals according to selected baseline characteristics – first cardiovascular hospitalisation or death from any cause:
Similar results were obtained on the incidence of cardiovascular hospitalisation with a risk reduction of 25.5% (p <0.0001).
During the course of the study, the number of deaths from any cause was comparable between the dronedarone (116/2,301) and placebo (139/2,327) groups.
Maintenance of sinus rhythm
In EURIDIS and ADONIS, a total of 1,237 patients with a prior episode of AF or AFL were randomised in an outpatient setting and treated with either dronedarone 400 mg twice daily (n=828) or placebo (n=409) on top of conventional therapies (including oral anticoagulants, beta-blockers, ACE inhibitors or AIIRAs, chronic antiplatelet agents, diuretics, statins, digitalis, and calcium antagonists). Patients had at least one ECG-documented AF/AFL episode during the last 3 months and were in sinus rhythm for at least one hour and were followed for 12 months. In patients who were taking amiodarone, an ECG was to be performed about 4 hours after the first administration to verify good tolerability. Other antiarrhythmic medicinal products had to be withdrawn for at least 5 plasma half-lives prior to the first administration. Patients ranged in age from 20 to 88 years, with the majority being Caucasian (97%), male (69%) patients. The most common co-morbidities were hypertension (56.8%) and structural heart disease (41.5%) including coronary heart disease (21.8%).
In the pooled data from EURIDIS and ADONIS as well as in the individual trials, dronedarone consistently delayed the time to first recurrence of AF/AFL (primary endpoint). As compared to placebo, dronedarone lowered the risk of first AF/AFL recurrence during the 12-month study period by 25% (p=0.00007). The median time from randomised to first AF/AFL recurrence in the dronedarone group was 116 days, i.e. 2.2-fold longer than in the placebo group (53 days).
The DIONYSOS study compared the efficacy and safety of dronedarone (400 mg twice daily) versus amiodarone (600 mg daily for 28 days, then 200 mg daily thereafter) over 6 months. A total of 504 patients with documented AF were randomised, 249 received dronedarone and 255 received amiodarone. Patients ranged in age from 28 to 90 years, 49% were more than 65 years old. The incidence of the primary efficacy endpoint defined as first recurrence of AF or premature study drug discontinuation for intolerance or lack of efficacy at 12 months was 75% in the dronedarone group and 59% in the amiodarone group (hazard ratio =1.59, log-rank p-value <0.0001). AF recurrence was 63.5% versus 42%, respectively. Recurrences of AF (including absence of conversion) were more frequent in the dronedarone group, whereas premature study drug discontinuations due to intolerance were more frequent in the amiodarone group. The incidence of the main safety endpoint defined as the occurrence of thyroid, hepatic, pulmonary, neurological, skin, eye or gastrointestinal specific events or premature study drug discontinuation following any adverse event was reduced by 20% in the dronedarone group compared to the amiodarone group (p=0.129). This reduction was driven by the occurrence of significantly fewer thyroid and neurological events and a trend for less skin or ocular events, and fewer premature study drug discontinuations compared to the amiodarone group. More gastrointestinal adverse events, mainly diarrhoea, were observed in the dronedarone group (12.9% versus 5.1%).
Patients with symptoms of heart failure at rest or with minimal exertion within the previous month prior, or who were hospitalised for heart failure during the previous month.
The ANDROMEDA study was conducted in 627 patients with left ventricular dysfunction, hospitalised with new or worsening heart failure and who had had at least one episode of shortness of breath on minimal exertion or at rest (NYHA class III or IV) or paroxysmal nocturnal dyspnoea within the month before admission. Patients ranged in age from 27 to 96 years, 68% were more than 65 years old. The study was stopped prematurely due to an observed imbalance of deaths in the dronedarone group [n=25 versus 12 (placebo), p=0.027] (see sections 4.3 and 4.4).
Patients with Permanent Atrial Fibrillation:
The PALLAS study was a randomised placebo-controlled study investigating the clinical benefit of dronedarone 400 mg BID on top of standard therapy in patients with permanent atrial fibrillation and additional risk factors (patients with congestive heart failure ~ 69%, coronary heart disease ~41%, prior stroke or TIA ~27%; LVEF ≤40% ~20.7% and patients ≥75 years with hypertension and diabetes ~18%). The study was prematurely stopped after randomization of 3149 patients (placebo =1577; dronedarone =1572) due to the significant increase in heart failure (placebo =33; dronedarone = 80; HR =2.49 (1.66-3.74)]; stroke [placebo = 8; dronedarone =17; HR =2.14 (0.92-4.96)] and cardiovascular death [placebo = 6; dronedarone =15; HR =2.53 (0.98-6.53)] (see sections 4.3 and 4.4).
Pharmacokinetic properties
Absorption
Following oral administration in fed condition, dronedarone is well absorbed (at least 70%). However due to presystemic first pass metabolism, the absolute bioavailability of dronedarone (given with food) is 15%. Concomitant intake of food increases dronedarone bioavailability by on average 2- to 4-fold.
After oral administration in fed conditions, peak plasma concentrations of dronedarone and the main circulating active metabolite (N-debutyl metabolite) are reached within 3 to 6 hours. After repeated administration of 400 mg twice daily, steady state is reached within 4 to 8 days of treatment and the mean accumulation ratio for dronedarone ranges from 2.6 to 4.5. The steady state mean dronedarone Cmax is 84-147 ng/ml and the exposure of the main N-debutyl metabolite is similar to that of the parent compound. The pharmacokinetics of dronedarone and its N-debutyl metabolite both deviate moderately from dose proportionality: a 2-fold increase in dose results in an approximate 2.5- to 3.0-fold increase with respect to Cmax and AUC.
Distribution
The in vitro plasma protein binding of dronedarone and its N-debutyl metabolite is 99.7% and 98.5% respectively and is not saturable. Both compounds bind mainly to albumin. After intravenous (IV) administration the volume of distribution at steady state (Vss) ranges from 1,200 to 1,400 L.
Biotransformation
Dronedarone is extensively metabolised, mainly by CYP 3A4 (see section 4.5). The major metabolic pathway includes N-debutylation to form the main circulating active metabolite followed by oxidation, oxidative deamination to form the inactive propanoic acid metabolite, followed by oxidation, and direct oxidation. Monoamine Oxidases contribute partially to the metabolism of the active metabolite of dronedarone (see section 4.5).
The N-debutyl metabolite exhibits pharmacodynamic activity but is 3 to 10-times less potent than dronedarone. This metabolite contributes to the pharmacological activity of dronedarone in humans.
Elimination
After oral administration, approximately 6% of the labelled dose is excreted in urine mainly as metabolites (no unchanged compound excreted in urine) and 84% are excreted in faeces mainly as metabolites. After intravenous administration the plasma clearance of dronedarone ranges from 130 to 150 L/h. The terminal elimination half-life of dronedarone is around 25-30 hours and that of its N-debutyl metabolite around 20-25 hours. In patients, dronedarone and its metabolite are completely eliminated from the plasma within 2 weeks after the end of a 400 mg twice daily-treatment.
Special populations
The pharmacokinetics of dronedarone in patients with AF is consistent with that in healthy subjects. Gender, age and weight are factors that influence the pharmacokinetics of dronedarone. Each of these factors has a limited influence on dronedarone.
Gender
In female patients, dronedarone exposures and its N-debutyl metabolite exposure are on average 1.3- to 1.9-fold higher as compared to male patients.
Elderly
Of the total number of subjects in clinical studies of dronedarone, 73% were 65 years of age and over and 34% were 75 years of age and over. In patients aged 65 years of age and over, dronedarone exposures are 23% higher in comparison with patients aged below 65 years of age.
Hepatic impairment
In subjects with moderate hepatic impairment, dronedarone unbound exposure is increased by 2-fold. That of the active metabolite is decreased by 47% (see section 4.2).
The effect of severe hepatic impairment on the pharmacokinetics of dronedarone was not assessed (see section 4.3).
Renal impairment
The effect of renal impairment on dronedarone pharmacokinetics has not been evaluated in a specific study. Renal impairment is not expected to modify the pharmacokinetics of dronedarone because no unchanged compound was excreted in urine and only approximately 6% of the dose was excreted in urine as metabolites (see section 4.2).
Preclinical safety data
Dronedarone had no genotoxic effects, based on one in vivo micronucleus test in mice and four in vitro tests.
In 2-year oral carcinogenicity studies, the highest dronedarone dose administered for 24 months was 70 mg/kg/day in rats and 300 mg/kg/day in mice.
Observations were increased incidence of mammary gland tumors in female mice, histiocytic sarcomas in mice and hemangiomas at the mesenteric lymph node level in rats, all at the highest tested dose only (corresponding to an exposure of 5 to 10 times that of the human therapeutic dose). Hemangiomas are not precancerous changes and do not transform into malignant hemangiosarcomas in either animals or man. None of these observations was considered relevant for humans.
In chronic toxicity studies, slight and reversible phospholipidosis (accumulation of foamy macrophages) was observed in mesenteric lymph nodes mainly in the rat. This effect is considered specific to this species and not relevant to humans.
Dronedarone caused marked effects on embryo-foetal development at high doses in rats, such as increased post-implantation losses, reduced foetal and placental weights, and external, visceral and skeletal malformations.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.