MYBULEN Capsule Ref.[51175] Active ingredients: Codeine Ibuprofen Paracetamol
Source: Health Products Regulatory Authority (ZA) Revision Year: 2022 Publisher: PHARMACARE LIMITED, Healthcare Park, Woodlands Drive, Woodmead, 2191
4.1. Therapeutic indications
MYBULEN CAPSULES are indicated for the relief of mild to moderate pain of inflammatory origin with or without fever for a maximum treatment period of 5 days.
4.2. Posology and method of administration
Posology
DO NOT EXCEED THE RECOMMENDED DOSE.
Use the lowest effective dose for the shortest possible duration of treatment.
Adults (over the age of 12 years)
Take one to two capsules 4 hourly.
Do not take more than 6 capsules in a 24 hour period.
Consult your healthcare provider if you require further treatment after 5 days.
MYBULEN CAPSULES should not be administered continuously for longer than 5 days as safety has not been established.
Paediatric population
Not recommended for children 12 years of age and younger.
Method of administration
For oral administration.
4.9. Overdose
Symptoms
The most frequently reported symptoms of overdose for the ingredients as contained in MYBULEN CAPSULES are mentioned below.
Ibuprofen
Most patients who have ingested significant amounts of ibuprofen, as in MYBULEN CAPSULES, will manifest symptoms within 4 to 6 hours.
Symptoms such as nausea, vomiting, abdominal pain, lethargy, blurred vision and drowsiness. Central nervous system (CNS) effects include headache, tinnitus, dizziness, convulsion, and loss of consciousness. Nystagmus, metabolic acidosis, hypothermia, renal effects, gastrointestinal bleeding, coma, apnoea, diarrhoea and depression of the CNS and respiratory system have also been reported.
Disorientation, excitation, fainting and cardiovascular toxicity, including hypotension, bradycardia and tachycardia have been reported. In cases of significant overdose, renal failure and liver damage are possible. Large overdoses are generally well tolerated when no other medicines are being taken.
In more serious poisoning, toxicity is seen in the central nervous system, manifesting as vertigo, dizziness, drowsiness, occasionally excitation and loss of consciousness or coma. Children may also develop myoclonic cramps. In serious poisoning metabolic acidosis may occur, hypothermia and hyperkalaemia may also occur, and the prothrombin time/INR may be prolonged, probably due to interference with the actions of circulating clotting factors. Respiratory depression and cyanosis may occur. Exacerbation of asthma is possible in asthmatics.
Paracetamol
Symptoms of paracetamol overdosage in the first 24 hours include pallor, nausea, vomiting, anorexia and possibly abdominal pain. Mild symptoms during the first two days of acute poisoning do not reflect the potential seriousness of the overdosage.
Liver damage may become apparent 12 to 48 hours, or later after ingestion of paracetamol, initially by elevation of the serum transaminase and lactic dehydrogenase activity, increased serum bilirubin concentrations and international normalised ratio (INR) (prolongation of the prothrombin time).
Liver damage may lead to encephalopathy, coma and death. Acute renal failure with acute tubular necrosis may develop even in the absence of severe liver damage. Abnormalities of glucose metabolism and metabolic acidosis may occur. Cardiac dysrhythmias have been reported. Cerebral oedema and non-specific myocardial depression have occurred.
Codeine phosphate
Symptoms of overdosage include excitement and, in children, convulsions may occur. Symptoms may result in central nervous system and respiratory depression with hypoxia, hypotension, shock, gastric hypomotility with ileus, and non-cardiogenic pulmonary oedema. The opiate intoxication syndrome is described as a triad of depressed level of consciousness, miotic pupils, and decreased frequency and depth of respirations.
Treatment
The following treatment is indicated for the ingredients as contained in MYBULEN CAPSULES.
Prompt treatment is essential as MYBULEN CAPSULES contains paracetamol.
Ibuprofen
Treatment should be symptomatic and supportive and include the maintenance of a clear airway and monitoring of cardiac and vital signs until stable. Within one hour of ingestion of a potentially toxic amount, oral administration of activated charcoal should be considered. Alternatively, in adults, gastric lavage should be considered within one hour of ingestion of a potentially life-threatening overdose.
Good urine output should be ensured.
Renal and liver function should be closely monitored.
Patients should be observed for at least four hours after ingestion of potentially toxic amounts. Frequent or prolonged convulsions should be treated with intravenous diazepam or lorazepam. Other measures may be indicated by the patient’s clinical condition.
If ibuprofen has already been absorbed, alkaline substances should be administered to promote the excretion of the acid ibuprofen in the urine.
Bronchodilators should be given for asthma. No specific antidote is available.
Paracetamol
Prompt treatment is essential. In the event of an overdosage, consult a doctor immediately, or take the person to a hospital directly. A delay in starting treatment may mean that the antidote is given too late to be effective. Evidence of liver damage is often delayed until after the time for effective treatment has lapsed.
Susceptibility to paracetamol toxicity is increased in patients who have taken repeated high doses (greater than 5 to 10 g/day) of paracetamol for several days, in chronic alcoholism, chronic liver disease, AIDS, malnutrition, and with the use of medicines that induce liver microsomal oxidation such as barbiturates, isoniazid, rifampicin, phenytoin and carbamazepine.
Although evidence is limited it is recommended that an adult person who has ingested 5 to 10 grams or more of paracetamol (or a child who has had more than 140 mg/kg) within the preceding four hours, should have the stomach emptied by lavage (emesis may be adequate for children) and a single dose of 50 g activated charcoal given via the lavage tube. Ingestion of amounts of paracetamol smaller than this may require treatment in patients susceptible to paracetamol poisoning (see above). In patients who are stuperose or comatose, endotracheal intubation should precede gastric lavage in order to avoid aspiration.
N-acetylcysteine should be administered to all cases of suspected overdose as soon as possible preferably within eight hours of overdosage, although treatment up to 36 hours after ingestion may still be of benefit, especially if more than 150 mg/kg of paracetamol was taken. An initial dose of 150 mg/kg N-acetylcysteine in 200 ml dextrose injection given intravenously over 15 minutes, followed by an infusion of 50 mg/kg in 500 ml dextrose injection over the next four hours, and then 100 mg/kg in 1 000 ml dextrose injection over the next sixteen hours. The volume of intravenous fluid should be modified for children.
Although the oral formulation is not the treatment of choice, 140 mg/kg dissolved in water as a 5% solution may be administered initially, followed by 70 mg/kg every four hours for seventeen doses.
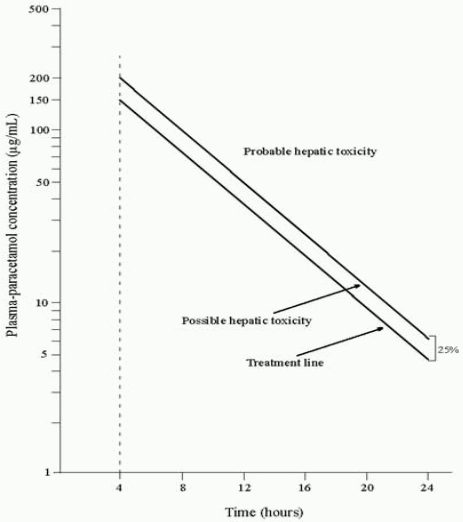
A plasma paracetamol level should be determined four hours after ingestion in all cases of suspected overdosage. Levels done before four hours, unless high, may be misleading. Patients at risk of liver damage, and hence requiring continued treatment with N-acetylcysteine, can be identified according to their plasma paracetamol level. The plasma paracetamol level can be plotted against time since ingestion in the treatment nomogram below. The nomogram should be used only in relation to a single acute ingestion.
Those whose plasma paracetamol levels are above the “normal treatment line”, should continue N-acetylcysteine treatment with 100 mg/kg IV over sixteen hours repeatedly until recovery. Patients with increased susceptibility to liver damage as identified above, should continue treatment if concentrations are above the “high risk treatment line”. Prothrombin index correlates best with survival.
Monitor all patients with significant ingestions for at least ninety-six hours.
Codeine phosphate
Treatment of overdosage is symptomatic and supportive.
Treatment is based on clinical presentation.
Plasma codeine levels are not clinically useful.
Support the respiratory and cardiovascular function.
Monitor arterial blood gases and/or pulse oximetry, pulmonary function tests and chest x-ray in patients with significant exposure.
Consider pre-hospital administration of activated charcoal as aqueous slurry in patients with a potentially toxic ingestion who are awake and able to protect their airway. Activated charcoal is most effective when administered within one hour of ingestion.
Use a minimum of 240 millilitres of water per 30 grams charcoal.
Optimum dose has not been established, but the usual dose is 25 to 100 grams in adults and adolescents; 25 to 50 grams in children aged 1 to 12 years (or 0,5 to 1 gram/kilogram body weight); and 1 gram/kilogram in infants up to 1 year old.
Consider naloxone as an antidote in patients with a decreased level of consciousness. The most frequently recommended initial naloxone dose for codeine overdose is 0,4 to 2 milligrams given as an intravenous bolus in both children and adults.
This dose can also be given subcutaneously in the absence of intravenous access, or intratracheally.
6.3. Shelf life
24 months.
6.4. Special precautions for storage
Store at or below 25°C in airtight containers.
Protect from moisture.
Keep in original packaging until required for use.
6.5. Nature and contents of container
10 capsules are packed in a clear polyvinylchloride, polyethylene, polyvinylidene chloride film sealed with an aluminium foil backing. The blister strips are packed into a unit cardboard carton together with a leaflet.
30 capsules are packed in a white polypropylene securitainer and sealed with a red or blue low-density polyethylene cap with a tamper evident seal together with a silica gel sachet or a white desiccant disc and a leaflet.
Not all packs and pack sizes are necessarily marketed.
6.6. Special precautions for disposal and other handling
No special requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.