OHTUVAYRE Inhalation suspension Ref.[110583] Active ingredients: Ensifentrine
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
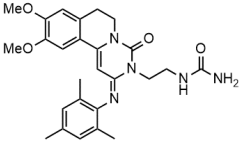
OHTUVAYRE (ensifentrine) is a sterile, yellow to pale yellow aqueous inhalation suspension of ensifentrine for oral inhalation. Ensifentrine, the active component of OHTUVAYRE, is an inhibitor of phosphodiesterases 3 and 4 (PDE3 and PDE4). The chemical name for ensifentrine is N-(2-{(2E)-9,10-dimethoxy-4-oxo-2-[(2,4,6-trimethylphenyl)imino]-6,7-dihydro-2H-pyrimido[6,1-a]isoquinolin3(4H)-yl}ethyl)urea; its structural formula is:
Ensifentrine has a molecular weight of 477.56 and its empirical formula is C26H31N5O4. Ensifentrine is a yellow to pale yellow crystalline powder which is practically insoluble in water.
OHTUVAYRE is supplied as 2.5 mL of sterile ensifentrine (1.2 mg/mL) suspension packaged in a unit-dose low-density polyethylene ampule overwrapped in a sealed foil pouch. Each unit-dose ampule contains 3 mg ensifentrine suspended in a pH 6.7 aqueous solution containing dibasic sodium phosphate, monobasic sodium phosphate, polysorbate 20, sodium chloride, sorbitan monolaurate and water for injection.
The ampule containing OHTUVAYRE should be shaken vigorously to ensure complete resuspension of the active ingredient immediately prior to administration by nebulization. Like all other nebulized treatments, the amount delivered to the lungs will depend on patient factors, the nebulization system used, and compressor performance.
Using the PARI LC Sprint jet nebulizer attached to a PARI Vios Pro compressor under in vitro conditions, the mean delivered dose from the mouthpiece was approximately 933 micrograms (31% of label claim) at a mean flow rate of approximately 5 liters per minute. The mean nebulization time was approximately 7 minutes. The mass median aerodynamic diameter (MMAD) of the nebulized particles/droplets using the PARI LC Sprint jet nebulizer attached to a PARI Vios Pro compressor (flow rate approximately 15 L per minute, nebulization time approximately 10 minutes) is 5.82 microns (geometric standard deviation = 1.97) with a typical fine particle dose (mass of aerosolized drug <5 microns) of approximately 614 micrograms, as determined using the Next Generation Impactor (NGI) method, based on USP <601>. OHTUVAYRE should only be administered via a standard jet nebulizer connected to an air compressor with an adequate airflow and equipped with a mouthpiece.
| Dosage Forms and Strengths |
|---|
|
Inhalation suspension: 3 mg/2.5 mL (1.2 mg/mL) of sterile, yellow to pale yellow, aqueous suspension in low-density polyethylene unit-dose ampules. |
| How Supplied |
|---|
|
OHTUVAYRE (ensifentrine) 3 mg/2.5 mL inhalation suspension is a sterile aqueous suspension in a unit-dose low-density polyethylene ampule. OHTUVAYRE is supplied in a carton of 60 unit-dose ampules (NDC 83034-003-60). Each ampule is overwrapped in a sealed foil pouch. The ampule containing OHTUVAYRE should be shaken vigorously to ensure complete resuspension of the active ingredient immediately prior to use. The used ampule and any residual content should be discarded after use. Manufactured for: Verona Pharma, Inc., 8529 Six Forks Road, Suite #400, Raleigh, NC 27615 |
Drugs
| Drug | Countries | |
|---|---|---|
| OHTUVAYRE | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.