OHTUVAYRE Inhalation suspension Ref.[110583] Active ingredients: Ensifentrine
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
Ensifentrine is a small molecule that is an inhibitor of the PDE3 and PDE4 enzymes. PDE3 primarily hydrolyzes the second-messenger molecule cyclic adenosine monophosphate (cAMP) but is also capable of hydrolyzing cyclic guanosine monophosphate (cGMP). PDE4 hydrolyzes cAMP only. Inhibition of PDE3 and PDE4 results in accumulation of intracellular levels of cAMP and/or cGMP, resulting in various downstream signalling effects.
12.2. Pharmacodynamics
Cardiac Electrophysiology
QTc interval prolongation was studied in a randomized, double-blind, placebo- and positivecontrolled, 4-period crossover study in 32 healthy subjects. At 3 times the maximum recommended dose, clinically significant QTc interval prolongation was not observed.
12.3. Pharmacokinetics
Exposure to ensifentrine increased approximately 1.4-fold greater than dose proportional following a dose 3 times the recommended dosage. Steady-state was attained by Day 3 following twice-daily dosing. Population pharmacokinetic analysis predicts accumulation of ensifentrine of 1.3 and 1.4-fold for Cmax and AUC in healthy subjects and 1.4 and 1.5-fold for Cmax and AUC in subjects with COPD. Population pharmacokinetic analysis indicates that relative bioavailability in subjects with COPD is approximately 35% lower when compared to healthy subjects. Exposure to ensifentrine was associated with high inter-subject variability.
Absorption
Following inhaled administration of OHTUVAYRE in healthy subjects and subjects with COPD, ensifentrine Cmax was attained around 0.6 to 1.5 hours after dosing.
A randomized, 2-period, cross-over study assessing systemic exposure following inhalation of 2 times the recommended dose of ensifentrine with and without charcoal block demonstrated that the majority of an inhaled dose (approximately 90%) is delivered to the lung from which it is absorbed.
Distribution
Apparent central and peripheral volume of distribution for ensifentrine in healthy subjects were 2700 L and 1820 L, respectively, as estimated in population PK analysis. In patients with COPD, apparent central and peripheral volumes were estimated as 8150 L and 5490 L, respectively.
In vitro plasma protein binding of ensifentrine is approximately 90%.
Elimination
Following twice-daily administration for 6 days, terminal elimination half-life ranged from 10.6 to 12.6 hours in healthy subjects and subjects with COPD (1.5 mg to 12 mg twice daily).
Metabolism
Following administration of a single nebulized dose, 8 times the recommended dose of ensifentrine, unchanged ensifentrine was identified as the major drug-related component in human plasma, accounting for 96 and 99% of the drug-related material identified in Tmax and time-normalized (0-24 h) plasma samples, respectively.
The primary metabolic routes for ensifentrine are oxidative (hydroxylation, O-demethylation) followed by conjugation (e.g., glucuronidation).
In vitro results indicate that, at physiologically relevant concentrations, ensifentrine was predominantly metabolized by CYP2C9 and to a lesser extent by CYP2D6.
Excretion
The majority of ensifentrine is excreted in feces. After a 3 mg nebulized dose, urinary elimination of unchanged ensifentrine was negligible (<0.3% of the dose).
Specific Populations
Population pharmacokinetic analysis showed no evidence of a clinically significant effect of demographic covariates such as age (18 to 80 years), sex (56% male), ethnicity (Hispanic, NonHispanic), race (white, black) and weight (42 to 180 kg) on ensifentrine pharmacokinetics.
Patients with Renal Impairment
A dedicated study with OHTUVAYRE evaluating the effect of renal impairment on the pharmacokinetics of ensifentrine was not conducted.
The effect of renal impairment on the exposure to ensifentrine for up to 24 weeks was evaluated in a population pharmacokinetic analysis. Estimated glomerular filtration rate (eGFR) varied from 25.5 to 191 mL/min representing a range of moderate to no renal impairment. While continuous covariates of renal function did not show a significant correlation with ensifentrine exposure, categorical characterization of renal function indicated a 25% mean reduction in the apparent clearance in subjects with moderate renal impairment. The pharmacokinetics of ensifentrine in severe renal impairment (creatine clearance <30 mL/min) or subjects with end-stage renal disease have not been evaluated.
Patients with Hepatic Impairment
The pharmacokinetics of ensifentrine were evaluated in subjects with moderate (Child-Pugh Class B) (N=10) to severe (Child-Pugh Class C) (N=2) hepatic impairment. Ensifentrine Cmax and AUCinf were approximately 2.3-fold and 2.2-fold higher in subjects with moderate hepatic impairment compared with healthy controls. Ensifentrine Cmax and AUCinf were approximately 1.2-fold and 2.3-fold higher in subjects with severe hepatic impairment compared with healthy controls (N=7). Population PK analysis did not identify markers of liver function (ALT, AST, bilirubin, and ALP) as a significant covariate for exposure of ensifentrine.
Drug Interaction Studies
Effect of Other Drugs on OHTUVAYRE
Clinical Studies:
Ensifentrine and Cytochrome P450: Ensifentrine Cmax and AUC0-inf were 1.4-fold and 1.6-fold higher; respectively, when a 3 mg single dose of OHTUVAYRE was concomitantly administered with CYP2C9 inhibitor fluconazole (200 mg twice daily).
In Vitro Studies:
Ensifentrine and Efflux Transporters: Ensifentrine is not a substrate of the efflux transporter P-glycoprotein (P-gp). Ensifentrine is a substrate of breast cancer resistance protein (BCRP).
Ensifentrine and Uptake Transporters: Ensifentrine is not a substrate of the uptake transporters OATP1B1 or OATP1B3.
Effect of OHTUVAYRE on Other Drugs
In Vitro Studies:
Ensifentrine and Cytochrome P450: At therapeutically relevant concentrations, ensifentrine does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4.
Ensifentrine and Efflux Transporters: At therapeutically relevant concentrations, ensifentrine is not an inhibitor of either BCRP or P-gp.
Ensifentrine and Uptake Transporters: Ensifentrine does not inhibit the transporters, OATP1B1, OATP1B3, OAT1, OAT3, OCT2, MATE1 or MATE2-K, at therapeutically relevant concentrations.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
A two-year inhalation study in Han Wistar rats and a 6-month oral study in Tg.rasH2 transgenic mice were conducted to assess the carcinogenic potential of ensifentrine. No evidence of tumorigenicity was observed in male and female rats at an exposure approximately 40 times the MRHDID. No evidence of tumorigenicity was observed in male and female Tg.rasH2 mice at oral doses up to 80 mg/kg/day, the highest dose tested.
Ensifentrine was negative for genotoxicity in the following assays: in vitro Ames test for bacterial gene mutation, in vivo comet test with rats, or in vivo micronucleus assay with mice.
In a male fertility study, ensifentrine was administrated to male rats at inhalation doses of 2, 6, and 16 mg/kg/day (4, 13, and 30 times the exposure at the MRHDID) for 10 weeks prior to mating to untreated females. Male rats had decreased sperm motility and increased abnormal sperm morphology at an inhalation dose of 16 mg/kg/day (approximately 30 times the exposure at the MRHDID). Decreased sperm counts in the testis were observed at all doses. Atrophy/degeneration in the testis and intraluminal germ cell debris in the epididymis were observed at doses of 6 (13 times the exposure at the MRHDID) and 16 mg/kg/day (30 times the exposure at the MRHDID). Additional adverse effects at 16 mg/kg/day on reproductive performance included decreased mating index and decreased fertility index. The sperm counts, sperm motility, and sperm morphology were reversible at the end of a 4-week treatment-free period. Atrophy/degeneration in the testis and intraluminal germ cell debris in the epididymis were not present at the end of a 4-week treatment-free period. In a female fertility study, ensifentrine was administrated to female rats at inhalation doses of up to 18 mg/kg/day from two weeks prior to mating to 7 days after mating. Ensifentrine had no effect on female fertility and reproductive performance indices up to 18 mg/kg/day (31 times the exposure at the MRHDID).
14. Clinical Studies
The efficacy of OHTUVAYRE was evaluated in two 24-week randomized, double-blind, placebocontrolled, parallel-group clinical trials (ENHANCE-1 [NCT04535986] and ENHANCE-2 [NCT04542057]). The two trials enrolled a total of 1553 adults with moderate to severe COPD.
ENHANCE-1 enrolled a total of 763 patients randomized 5:3 to receive 3 mg of OHTUVAYRE administered by oral inhalation via standard jet nebulizer such as PARI LC Sprint [see Description (11)], or placebo. Patients in ENHANCE-1 had a mean age of 65 years (range: 41 to 80 years), were 58% male, 90% White, 3% Black/African American, 3% Asian, 0.1% Other race, and 3% Hispanic or Latino ethnicity. Patients had a mean smoking history of 41 pack-years and 57% were current smokers, and 25% of patients reported exacerbations of COPD within the 15 months prior to the study. At screening, the mean post-bronchodilator percent predicted FEV1 was 52% (range: 27% to 85%), and the mean post-bronchodilator FEV1/FVC ratio was 0.52 (range: 0.22 to 0.71). In addition, 68% of patients were taking concurrent therapy: 30% taking concurrent LAMA, 18% taking concurrent LABA, and 20% taking concurrent LABA/ICS therapy throughout the trial.
ENHANCE-2 enrolled a total of 790 patients randomized 5:3 to receive 3 mg of OHTUVAYRE twice daily administered by oral inhalation via a standard jet nebulizer such as PARI LC Sprint [see Description (11)] or placebo. Patients in ENHANCE-2 had a mean age of 65 years (range: 40 to 80 years), were 52% female, 95% White, 4% Black/African American, 0.3% Asian, 0.5% Other race, and 5% Hispanic or Latino ethnicity. Patients had a mean smoking history of 42 pack-years and 55% were current smokers, and 21% of patients reported exacerbations of COPD within the 15 months prior to the study. At screening, the mean post-bronchodilator percent predicted FEV1 was 51% (range: 23% to 81%), and the mean post-bronchodilator FEV1/FVC ratio was 0.52 (range: 0.24 to 0.71). In addition, 55% of patients were taking concurrent therapy: 33% taking concurrent LAMA, 7% taking concurrent LABA, and 15% taking concurrent LABA/ICS therapy throughout the trial.
The primary endpoint for ENHANCE-1 and ENHANCE-2 was the change from baseline in FEV1 AUC0-12h post dose at Week 12. In both trials, OHTUVAYRE demonstrated a statistically significant improvement in FEV1 AUC0-12h compared to placebo. Refer to Table 2 for the primary endpoint results.
Table 2. Least Squares (LS) Mean Change from Baseline in FEV1 AUC0-12h (mL) at Week 12 in ENHANCE-1 and ENHANCE-2:
| ENHANCE-1 | ENHANCE-2 | |||
|---|---|---|---|---|
| OHTUVAYRE (N=479) | Placebo (N=284) | OHTUVAYRE (N=499) | Placebo (N=291) | |
| n | 477 | 282 | 498 | 291 |
| LS Mean (95% CI) | 61 (25, 97) | -26 (-64, 13) | 48 (30, 66) | -46 (-70, -22) |
| LS Mean Difference from Placebo (95% CI) | 87 (55, 118) | - | 94 (65, 124) | - |
| p-value | <0.0001 | - | <0.0001 | - |
CI, confidence interval; LS, least squares; N = number of all enrolled patients; n = number of patients who received at least one dose of study drug and had non-missing baseline FEV1 value.
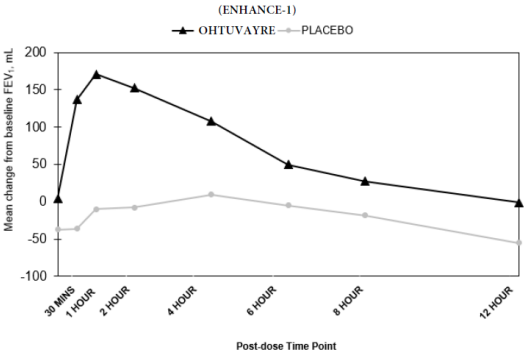
In ENHANCE-1 and ENHANCE-2, serial spirometry was performed over 12 hours in all patients at baseline and Week 12. Serial spirometry data for ENHANCE-1 at Week 12 are shown in Figure 1.
Figure 1. Mean FEV1 (mL) Change from Baseline over 12 hours at Week 12 (ENHANCE-1):
Trough FEV1 was defined as the last FEV1 value collected prior to the morning dose. The mean morning trough FEV1 improvement at Week 12 relative to placebo was 35 mL (95% CI: 14, 68) and 49 mL (95% CI: 19, 80) in ENHANCE-1 and ENHANCE-2, respectively, which was statistically significant in ENHANCE-1, and not statistically significant in ENHANCE-2 due to failure higher in the testing hierarchy.
Health-Related Quality of Life
The St. George's Respiratory Questionnaire (SGRQ) was assessed in ENHANCE-1 and ENHANCE2. In ENHANCE-1, the SGRQ responder rate (defined as an improvement in score of 4 or more as threshold) for OHTUVAYRE at Week 24 was 58.2% compared to 45.9% for placebo [Odds Ratio: 1.49; 95% CI: 1.07, 2.07]. In ENHANCE-2, the SGRQ responder rate for OHTUVAYRE at Week 24 was 45.4% compared to 50.3% for placebo [Odds Ratio: 0.92; 95% CI: 0.66, 1.29].
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.