ONYDA Extended-release oral suspension Ref.[110276] Active ingredients: Clonidine
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
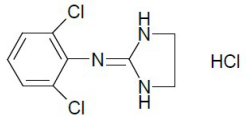
ONYDA XR contains clonidine hydrochloride, a centrally acting alpha2-adrenergic agonist. Clonidine hydrochloride is an imidazoline derivative and exists as a mesomeric compound. The chemical name is 2-[(2,6-dichlorophenyl)imino]imidazolidine hydrochloride. The following is the structural formula:
The molecular formula of clonidine hydrochloride is C9H9Cl2N3•HCl and the molecular weight is 266.5. The pKa is 8.05.
Clonidine hydrochloride is an odorless, bitter, white to almost white, crystalline powder soluble in water and alcohol. The pH of a 5% solution in water is between 3.5 and 5.5.
ONYDA XR is an extended-release suspension for oral administration. Each mL of ONYDA XR contains 0.09 mg clonidine equivalent to 0.1 mg clonidine hydrochloride (0.095 mg clonidine hydrochloride complexed with sodium polystyrene sulfonate and 0.005 mg clonidine hydrochloride). The pH of ONYDA XR is between 2.8 and 4.
The inactive ingredients are anhydrous citric acid, edetate disodium, glycerin, modified starch, methylparaben, orange flavor, polyvinyl acetate dispersion 30%, povidone, polysorbate 80, propylparaben, purified water, sucrose, sodium polystyrene sulfonate, triacetin, xanthan gum.
| Dosage Forms and Strengths |
|---|
|
Extended-release oral suspension: Light beige to tan viscous suspension containing 0.1 mg clonidine hydrochloride per mL. |
| How Supplied |
|---|
|
ONYDA XR (clonidine hydrochloride) extended-release oral suspension 0.1 mg/mL is a light beige to tan viscous suspension. ONYDA XR is supplied in 120 mL bottles with a child-resistant closure and is packaged in a carton with two oral dosing dispensers and two press in bottle adapters (NDC 24478-148-02). Manufactured by/Distributed by: Tris Pharma, Inc., Monmouth Junction, NJ 08852, www.trispharma.com |
Drugs
| Drug | Countries | |
|---|---|---|
| ONYDA | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.