ONYDA Extended-release oral suspension Ref.[110276] Active ingredients: Clonidine
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
Clonidine stimulates alpha2-adrenergic receptors in the brain. Clonidine is not a central nervous system stimulant. The mechanism of action of clonidine in ADHD is not known.
12.2. Pharmacodynamics
Clonidine is a known antihypertensive agent. By stimulating alpha2-adrenergic receptors in the brain stem, clonidine reduces sympathetic outflow from the central nervous system and decreases peripheral resistance, renal vascular resistance, heart rate, and blood pressure.
12.3. Pharmacokinetics
Immediate-release clonidine hydrochloride, extended-release clonidine hydrochloride tablets, and ONYDA XR have different pharmacokinetic characteristics. Dose substitution on a milligram for milligram basis will result in differences in exposures [see Dosage and Administration (2.3)].
Absorption
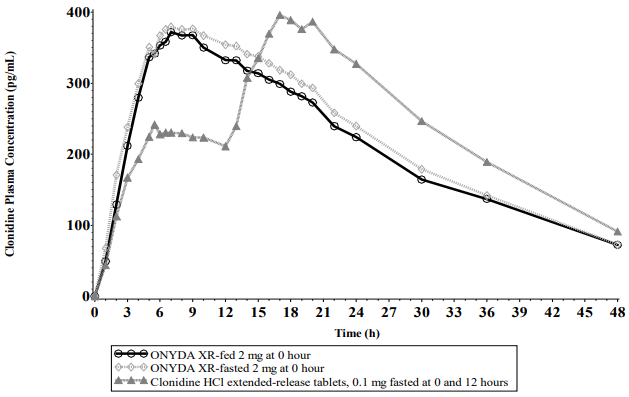
Following a single 0.2 mg dose of ONYDA XR in 20 healthy adult subjects under fasting conditions in a crossover study, the median (range) time to peak plasma concentrations (Tmax) for clonidine was 7.50 (4–17) hours after dosing. Peak concentration (Cmax) was 95.6% of the Cmax of clonidine extended-release tablet 0.1 mg administered at 0 and 12 hours under fasting conditions. The relative bioavailability of ONYDA XR compared with an equal dose of clonidine extended-release tablet was 96.1%.
After oral administration of 0.2 mg of ONYDA XR once daily over 5 days under fasted conditions in healthy adult subjects, the peak steady state plasma concentration (Cmax.ss) was 107.9%, and steady state relative bioavailability (AUCt,ss) was 97.7% compared with 0.1 mg of clonidine extended-release tablet administered twice daily under fasting conditions. The minimum concentration at steady state (Cmin,ss) of ONYDA XR was about 26% lower than that of the equal dose of clonidine extended-release tablet.
Following oral administration of an immediate release formulation in healthy adult subjects, plasma clonidine concentration peaks in approximately 3 to 5 hours.
A comparison across studies suggests that the Cmax is 50% lower for clonidine hydrochloride extended-release tablets compared to immediate-release clonidine hydrochloride.
Effect of Food
Food had no effect on plasma exposures of clonidine after administration of ONYDA XR (see Figure 1).
Figure 1. Mean Clonidine Concentration-Time Profiles After Single Dose Administration:
Elimination
The plasma half-life of immediate-release clonidine ranges from 12 to 16 hours. The half-life increases up to 41 hours in patients with severe impairment of renal function.
Metabolism
About 50% of the absorbed dose is metabolized in the liver.
Excretion
Following oral administration about 40% to 60% of the absorbed dose is recovered in the urine as unchanged drug in 24 hours. Although studies of the effect of renal impairment and studies of clonidine excretion have not been performed with ONYDA XR, results are expected to be similar to those of the immediate-release formulation.
Specific Populations
Pediatric patients
Plasma clonidine concentrations in pediatric patients 6 to 17 years (0.1 mg twice daily and 0.2 mg twice daily of clonidine hydrochloride extended-release tablets) with ADHD are greater than those of adults with hypertension, with pediatric patients 6 to 17 years receiving higher doses on a mg/kg basis. Body weight normalized clearance (CL/F) in pediatric patients aged 6 to 17 years was higher than CL/F observed in adults with hypertension. Clonidine concentrations in plasma increased with increases in dose over the dose range of 0.2 to 0.4 mg/day. Clonidine CL/F was independent of dose administered over the 0.2 to 0.4 mg/day dose range. Clonidine CL/F appeared to decrease slightly with increases in age over the range of 6 to 17 years, and females had a 23% lower CL/F than males. The incidence of “sedation-like” events (somnolence and fatigue) appeared to be independent of clonidine dose or concentration within the studied dose range in the titration study. Results from the add-on study showed that clonidine CL/F was 11% higher in patients who were receiving methylphenidate and 44% lower in those receiving amphetamine compared to subjects not on adjunctive therapy.
Drug Interaction Studies
Alcohol
In an in vitro alcohol-induced dose dumping study, a significantly faster and more variable ONYDA XR drug release was observed in the presence of 20% alcohol, but not with 5% or 10% alcohol, when compared to 0% alcohol.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Clonidine hydrochloride was not carcinogenic when administered in the diet of rats (for up to 132 weeks) or mice (for up to 78 weeks) at doses of up to 1,620 (male rats), 2,040 (female rats), or 2,500 (mice) mcg/kg/day. These doses are approximately 20, 25, and 15 times, respectively, the maximum recommended human dose (MRHD) of 0.4 mg/day on a mg/m² basis.
Mutagenesis
There was no evidence of genotoxicity in the Ames test for mutagenicity or mouse micronucleus test for clastogenicity.
Impairment of Fertility
In a reproduction study fertility of female rats appeared to be adversely affected at dose levels of 500 and 2,000 mcg/kg/day (10 and 40 times the MRHD on a mg/m² basis). Lower doses have not been adequately evaluated and a no adverse effect level could not be established.
14. Clinical Studies
The efficacy of ONYDA XR for the treatment of ADHD in pediatric patients 6 years of age and older is based upon adequate and well-controlled studies of clonidine hydrochloride extendedrelease tablets (referred to as “clonidine hydrochloride extended-release” in this section). The efficacy results of these adequate and well-controlled studies of clonidine hydrochloride extended-release tablets are presented below.
Efficacy of clonidine hydrochloride extended-release in the treatment of ADHD was established in pediatric patients 6 to 17 years in:
- One short-term, placebo-controlled monotherapy trial (Study 1)
- One short-term adjunctive therapy to psychostimulants trial (Study 2)
- One randomized withdrawal trial as monotherapy (Study 3)
Short-term Monotherapy and Adjunctive Therapy to Psychostimulant Studies for ADHD
The efficacy of clonidine hydrochloride extended-release in the treatment of ADHD was established in 2 (one monotherapy and one adjunctive therapy) placebo-controlled trials in pediatric patients aged 6 to 17 years, who met DSM-IV criteria of ADHD hyperactive or combined hyperactive/inattentive subtypes. Signs and symptoms of ADHD were evaluated using the investigator administered and scored ADHD Rating Scale-IV-Parent Version (ADHDRS-IV) total score including hyperactive/impulsivity and inattentive subscales. Study 1 was an 8-week randomized, double-blind, placebo-controlled, fixed dose study of pediatric patients 6 to 17 years (N=236) with a 5-week primary efficacy endpoint. Patients were randomly assigned to one of the following three treatment groups: clonidine hydrochloride extended-release 0.2 mg/day (N=78), clonidine hydrochloride extended-release 0.4 mg/day (N=80), or placebo (N=78). Dosing for the clonidine hydrochloride extended-release groups started at 0.1 mg/day and was titrated in increments of 0.1 mg/week to their respective dose (as divided doses). Patients were maintained at their dose for a minimum of 2 weeks before being gradually tapered down to 0.1 mg/day at the last week of treatment. At both doses, improvements in ADHD symptoms were statistically significantly superior in clonidine hydrochloride extended-release-treated patients compared with placebo-treated patients at the end of 5 weeks as measured by the ADHDRS-IV total score (Table 6). Study 2 was an 8-week randomized, double-blind, placebo-controlled, flexible dose study in pediatric patients 6 to 17 years (N=198) with a 5-week primary efficacy end point. Patients had been treated with a psychostimulant (methylphenidate or amphetamine) for four weeks with inadequate response. Patients were randomly assigned to one of two treatment groups: clonidine hydrochloride extended-release adjunct to a psychostimulant (N=102) or psychostimulant alone (N=96). The clonidine hydrochloride extended-release dose was initiated at 0.1 mg/day and doses were titrated in increments of 0.1 mg/week up to 0.4 mg/day, as divided doses, over a 3-week period based on tolerability and clinical response. The dose was maintained for a minimum of 2 weeks before being gradually tapered to 0.1 mg/day at the last week of treatment. ADHD symptoms were statistically significantly improved in clonidine hydrochloride extended-release plus stimulant group compared with the stimulant alone group at the end of 5 weeks as measured by the ADHDRS-IV total score (Table 6).
Table 6. Short-Term Trials:
| Study Number | Treatment Group | Primary Efficacy Measure: ADHDRS-IV Total Score | ||
|---|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Differencea (95% CI) | ||
| Study 1 | Clonidine hydrochloride extended-release tablets (0.2 mg/day) | 43.8 (7.47) | -15.0 (1.38) | -8.5 (-12.2, -4.8) |
| Clonidine hydrochloride extended-release tablets (0.4 mg/day) | 44.6 (7.73) | -15.6 (1.33) | -9.1 (-12.8, -5.5) | |
| Placebo | 45.0 (8.53) | -6.5 (1.35) | ---------- | |
| Study 2 | Clonidine hydrochloride extended-release tablets (0.4 mg/day) + Psychostimulant | 38.9 (6.95) | -15.8 (1.18) | -4.5 (-7.8, -1.1) |
| Psychostimulant alone | 39.0 (7.68) | -11.3 (1.24) | ---------- | |
a Difference (drug minus placebo) in least-squares mean change from baseline. SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
Maintenance Monotherapy for ADHD
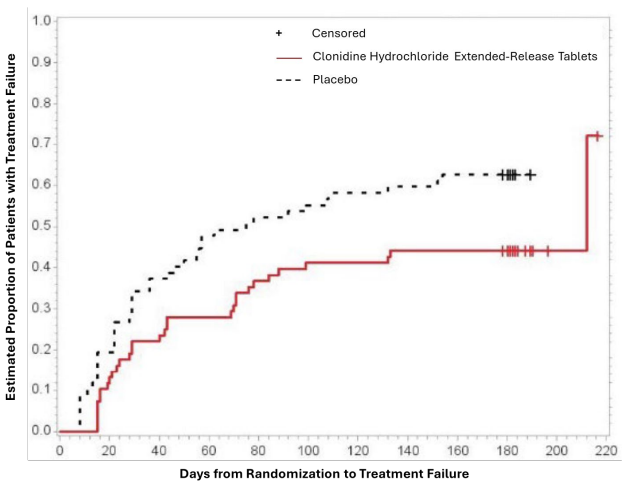
Study 3 was a double-blind, placebo-controlled, randomized-withdrawal study in pediatric patients 6 to 17 years (n=253) with DSM-IV-TR diagnosis of ADHD. The study consisted of a 10-week, open-label phase (4 weeks of dose optimization and 6 weeks of dose maintenance), a 26-week double-blind phase, and a 4-week taper-down and follow-up phase. All patients were initiated at 0.1 mg/day and increased at weekly intervals in increments of 0.1 mg/day until reaching personalized optimal dose (0.1, 0.2, 0.3 or 0.4 mg/day, as divided doses). Eligible patients had to demonstrate treatment response as defined by ≥30% reduction in ADHD-RS-IV total score and a Clinical Global Impression-Improvement score of 1 or 2 during the open label phase. Patients who sustained treatment response (n=135) until the end of the open label phase were randomly assigned to one of the two treatment groups, clonidine hydrochloride extendedrelease (N=68) and Placebo (N=67), to evaluate the long-term efficacy of maintenance dose of clonidine hydrochloride extended-release in the double-blind phase. The primary efficacy endpoint was the percentage of patients with treatment failure defined as a ≥30% increase (worsening) in ADHD-RS-IV total score and ≥2 points increase (worsening) in Clinical Global Impression – Severity Scale in 2 consecutive visits or early termination for any reason. A total of 73 patients experienced treatment failure in the double-blind phase: 31 patients (45.6%) in the clonidine hydrochloride extended-release group and 42 patients (62.7%) in the placebo group, with a statistically significant difference in the primary endpoint favoring clonidine (Table 7). The cumulative proportion of patients with treatment failure over time during the double-blind phase is displayed in Figure 2.
Table 7. Treatment Failure: Double-Blind Full Analysis Set (Study 3):
| Study 3 | Double-Blind Full Analysis Set | |
|---|---|---|
| Clonidine Hydrochloride Extended-Release Tablets | Placebo | |
| Number of patients | 68 | 67 |
| Number of treatment failures | 31 (45.6%) | 42 (62.7%) |
| Basis of Treatment Failure | ||
| Clinical criteriaa,b | 11 (16.2%) | 9 (13.4%) |
| Lack of efficacyc | 1 (1.5%) | 3 (4.5%) |
| Withdrawal of informed assent/consent | 4 (5.9%) | 20 (29.9%) |
| Other early terminations | 15 (22.1%) | 10 (14.9%) |
ADHD-RS-IV = Attention Deficit Hyperactivity Disorder-Rating Scale-4th edition; CGI-S = Clinical Global Impression-Severity
a At the same 2 consecutive visits a (1) 30% or greater reduction in ADHD-RS-IV, and (2) 2-point or more increase in CGI-S.
b Two patients (1 placebo and 1 clonidine hydrochloride extended-release tablets) withdrew consent, but met the clinical criteria for treatment failure.
c Three patients (all placebo) discontinued the study due to treatment failure, but met only the criterion for ADHD-RS-IV.
Figure 2. Kaplan-Meier Estimation of Cumulative Proportion of Pediatric Patients (6 to 17 Years) with Treatment Failure (Study 3):
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.