PEDMARQSI Solution for infusion Ref.[50987] Active ingredients: Thiosulfate
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Fennec Pharmaceuticals (EU) Limited, Block A, 5th Floor, The Atrium, Blackthorn Road, Sandyford, Dublin 18, Ireland
4.1. Therapeutic indications
Pedmarqsi is indicated for the prevention of ototoxicity induced by cisplatin chemotherapy in patients 1 month to <18 years of age with localised, non-metastatic, solid tumours.
4.2. Posology and method of administration
Pedmarqsi is intended for hospital use only, under the supervision of an appropriately qualified physician.
Posology
The recommended dose of sodium thiosulfate for the prevention of cisplatin-induced ototoxicity is weight based and normalised to body surface area according to the table below:
| Body Weight | Dose | Volume |
|---|---|---|
| >10 kg | 12.8 g/m² | 160 mL/m² |
| 5 to 10 kg | 9.6 g/m² | 120 mL/m² |
| <5 kg | 6.4 g/m² | 80 mL/m² |
Pre-treatment with antiemetics is recommended to reduce the incidence of nausea and vomiting (see section 4.4).
Special populations
Preterm and term newborn infants from birth to less than 1 month of age
Sodium thiosulfate is contraindicated in preterm and term newborn infants from birth to less than 1 month of age (see sections 4.3 and 4.4).
Renal impairment
No dose adjustment is recommended for patients with renal impairment (see section 5.2). Due to the sodium content of sodium thiosulfate, there is an increased risk of adverse reactions in patients with renal impairment (see section 4.4).
Hepatic impairment
No dose adjustment is recommended for patients with hepatic impairment (see section 5.2).
Method of administration
For intravenous use.
Due to the hypertonic formulation, administration through a central vein is recommended.
For single use only.
Sodium thiosulfate is administered as a 15-minute infusion.
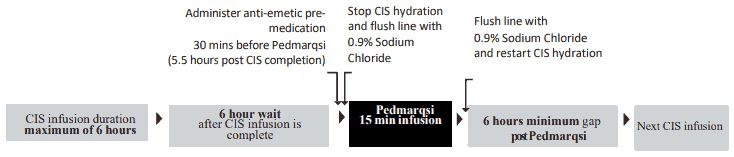
Time of administration in relation to cisplatin
The timing of sodium thiosulfate administration relative to cisplatin chemotherapy is critical.
If sodium thiosulfate is administered:
- Less than 6 hours after end of cisplatin infusion: may reduce cisplatin efficacy against the tumour
- More than 6 hours after end of cisplatin infusion: may not be effective in preventing ototoxicity
Only use sodium thiosulfate following cisplatin infusion duration of 6 hours or less. Do not use sodium thiosulfate if:
- Cisplatin infusion exceeds 6 hours, or
- A subsequent cisplatin infusion is planned within 6 hours
When cisplatin is administered on consecutive days, ensure a minimum 6-hour gap after sodium thiosulfate infusion before a subsequent cisplatin infusion is given.
After end of cisplatin infusion:
- Provide highly effective multi-agent intravenous antiemetic therapy 30 minutes prior to administration of sodium thiosulfate i.e. 5.5 hours after completion of cisplatin infusion
- This medicinal product is a ready to use solution for infusion
- Prepare the required mL of sodium thiosulfate, 80 mg/mL, in a syringe or add to an empty, sterile infusion bag
- Stop cisplatin hydration fluid and flush line with sodium chloride 0.9%
- Infuse sodium thiosulfate over 15 minutes (6 hours after completion of cisplatin infusion)
- Flush line with sodium chloride 0.9% and restart the cisplatin hydration immediately afterwards
CIS = cisplatin
4.9. Overdose
Excessive doses of sodium thiosulfate may be expected to produce severe nausea and vomiting as well as electrolyte imbalance, changes to blood pressure and acidosis. Treatment of an overdose should consist of general supportive measures including administration of fluids and observation of the clinical status of the patient. There is no specific antidote for overdose with sodium thiosulfate.
6.3. Shelf life
2 years.
From a microbiological point of view, the product should be used immediately after opening. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2°C-8°C.
Chemical and physical in-use stability has been demonstrated for 24 hours at controlled room temperature for product stored in polyvinyl chloride, ethylene vinyl acetate and polyolephine intravenous bags.
6.4. Special precautions for storage
This medicinal product does not require any special storage conditions.
For storage conditions after first opening of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Type I, 100 mL, clear glass vials sealed with a chlorinated butyl rubber stopper and an aluminium flipoff overseal. Each vial contains 100 mL of solution for infusion.
Vials are supplied in cartons of 1 vial pack.
6.6. Special precautions for disposal and other handling
This medicinal product is a sterile and ready to use solution for infusion.
Each vial is intended for single use only, and any unused solution should be discarded.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.