PLAVIX Film-coated tablet Ref.[10598] Active ingredients: Clopidogrel
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Clopidogrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.
12.2. Pharmacodynamics
Clopidogrel must be metabolized by CYP450 enzymes to produce the active metabolite that inhibits platelet aggregation. The active metabolite of clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet P2Y12 receptor and the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex, thereby inhibiting platelet aggregation. This action is irreversible. Consequently, platelets exposed to clopidogrel’s active metabolite are affected for the remainder of their lifespan (about 7 to 10 days). Platelet aggregation induced by agonists other than ADP is also inhibited by blocking the amplification of platelet activation by released ADP.
Dose-dependent inhibition of platelet aggregation can be seen 2 hours after single oral doses of Plavix. Repeated doses of 75 mg Plavix per day inhibit ADP-induced platelet aggregation on the first day, and inhibition reaches steady state between Day 3 and Day 7. At steady state, the average inhibition level observed with a dose of 75 mg Plavix per day was between 40% and 60%. Platelet aggregation and bleeding time gradually return to baseline values after treatment is discontinued, generally in about 5 days.
Geriatric Patients
Elderly (≥75 years) and young healthy subjects had similar effects on platelet aggregation.
Renally Impaired Patients
After repeated doses of 75 mg Plavix per day, patients with severe renal impairment (creatinine clearance from 5 to 15 mL/min) and moderate renal impairment (creatinine clearance from 30 to 60 mL/min) showed low (25%) inhibition of ADP-induced platelet aggregation.
Hepatically Impaired Patients
After repeated doses of 75 mg Plavix per day for 10 days in patients with severe hepatic impairment, inhibition of ADP-induced platelet aggregation was similar to that observed in healthy subjects.
Gender
In a small study comparing men and women, less inhibition of ADP-induced platelet aggregation was observed in women.
12.3. Pharmacokinetics
Clopidogrel is a prodrug and is metabolized to a pharmacologically active metabolite and inactive metabolites.
Absorption
After single and repeated oral doses of 75 mg per day, clopidogrel is rapidly absorbed. Absorption is at least 50%, based on urinary excretion of clopidogrel metabolites.
Effect of food
Plavix can be administered with or without food. In a study in healthy male subjects when Plavix 75 mg per day was given with a standard breakfast, mean inhibition of ADP-induced platelet aggregation was reduced by less than 9%. The active metabolite AUC0–24 was unchanged in the presence of food, while there was a 57% decrease in active metabolite Cmax. Similar results were observed when a Plavix 300 mg loading dose was administered with a high-fat breakfast.
Metabolism
Clopidogrel is extensively metabolized by two main metabolic pathways: one mediated by esterases and leading to hydrolysis into an inactive carboxylic acid derivative (85% of circulating metabolites) and one mediated by multiple cytochrome P450 enzymes. Cytochromes first oxidize clopidogrel to a 2-oxo-clopidogrel intermediate metabolite. Subsequent metabolism of the 2-oxo-clopidogrel intermediate metabolite results in formation of the active metabolite, a thiol derivative of clopidogrel. The active metabolite is formed mostly by CYP2C19 with contributions from several other CYP enzymes, including CYP1A2, CYP2B6 and CYP3A. The active thiol metabolite binds rapidly and irreversibly to platelet receptors, thus inhibiting platelet aggregation for the lifespan of the platelet.
The Cmax of the active metabolite is twice as high following a single 300 mg clopidogrel loading dose as it is after four days of 75 mg maintenance dose. Cmax occurs approximately 30 to 60 minutes after dosing. In the 75 to 300 mg dose range, the pharmacokinetics of the active metabolite deviates from dose proportionality: 4-fold the dose results in 2.0-fold and 2.7-fold the Cmax and AUC, respectively.
Elimination
Following an oral dose of 14C-labeled clopidogrel in humans, approximately 50% of total radioactivity was excreted in urine and approximately 46% in feces over the 5 days post dosing. After a single, oral dose of 75 mg, clopidogrel has a half-life of approximately 6 hours. The half-life of the active metabolite is about 30 minutes.
Drug Interactions
Effect of other drugs on Plavix
Clopidogrel is metabolized to its active metabolite in part by CYP2C19. Concomitant use of certain inhibitors of this enzyme results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition.
Proton pump inhibitors (PPI):
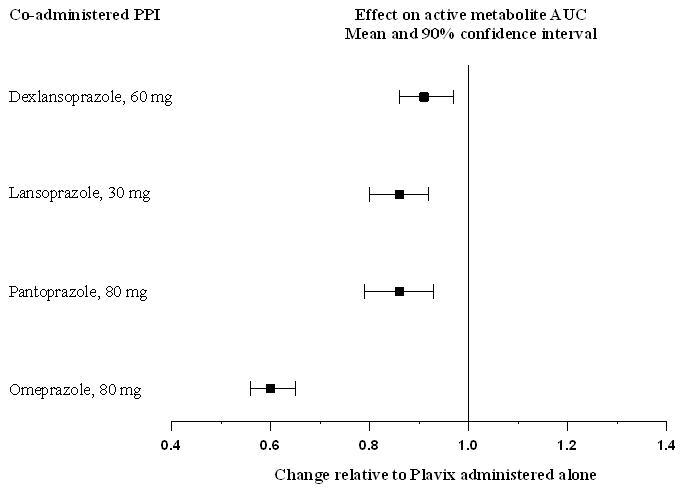
The effect of proton pump inhibitors (PPI) on the systemic exposure to the clopidogrel active metabolite following multiple doses of Plavix 75 mg evaluated in dedicated drug interaction studies is presented in Figure 1.
Figure 1. Exposure to Clopidogrel Active Metabolite Following Multiple Doses of Plavix 75 mg Alone or with Proton Pump Inhibitors (PPIs):
Pharmacodynamic and pharmacokinetic parameters measured in these studies showed that the interaction was highest with omeprazole and least with dexlansoprazole.
Opioids:
Coadministration of 5 mg intravenous morphine with 600 mg loading dose of clopidogrel in healthy adults decreased the AUC and Cmax of clopidogrel’s thiol metabolites by 34%. Mean platelet aggregation was higher up to 2 to 4 hours with morphine coadministration.
Effect of Plavix on other drugs
In vitro studies have shown that the glucuronide metabolite of clopidogrel is a strong inhibitor of CYP2C8. Concomitant administration of repaglinide with Plavix increased the systemic exposure to repaglinide (AUC0–∞) by 5.1-fold following the loading dose (300 mg) and by 3.9-fold on day 3 of the maintenance dose (75 mg) of Plavix [see Drug Interactions (7.6)].
12.5. Pharmacogenomics
CYP2C19 is involved in the formation of both the active metabolite and the 2-oxo-clopidogrel intermediate metabolite. Clopidogrel active metabolite pharmacokinetics and antiplatelet effects, as measured by ex vivo platelet aggregation assays, differ according to CYP2C19 genotype. Patients who are homozygous for nonfunctional alleles of the CYP2C19 gene are termed “CYP2C19 poor metabolizers”. Approximately 2% of White and 4% of Black patients are poor metabolizers; the prevalence of poor metabolism is higher in Asian patients (e.g., 14% of Chinese). Tests are available to identify patients who are CYP2C19 poor metabolizers.
A crossover study in 40 healthy subjects, 10 each in the four CYP2C19 metabolizer groups, evaluated pharmacokinetic and antiplatelet responses using 300 mg followed by 75 mg per day and 600 mg followed by 150 mg per day, each for a total of 5 days. Decreased active metabolite exposure and diminished inhibition of platelet aggregation were observed in the poor metabolizers as compared to the other groups.
Table 3. Active Metabolite Pharmacokinetics and Antiplatelet Responses by CYP2C19 Metabolizer Status:
| Dose | Poor (n=10) | Intermediate* (n=10) | Normal (n=10) | Ultrarapid† (n=10) | |
|---|---|---|---|---|---|
| Cmax (ng/mL) | 300 mg (24 h) | 11 (4) | 23 (11) | 32 (21) | 24 (10) |
| 600 mg (24 h) | 17 (6) | 39 (23) | 44 (27) | 36 (13) | |
| 75 mg (Day 5) | 4 (1) | 12 (5) | 13 (7) | 12 (6) | |
| 150 mg (Day 5) | 7 (2) | 18 (7) | 19 (5) | 16 (9) | |
| IPA (%)‡ | 300 mg (24 h) | 24 (26) | 37 (21) | 39 (28) | 40 (21) |
| 600 mg (24 h) | 32 (25) | 56 (22) | 49 (23) | 51 (28) | |
| 75 mg (Day 5) | 37 (23) | 60 (18) | 58 (19) | 56 (13) | |
| 150 mg (Day 5) | 61 (14) | 74 (14) | 73 (9) | 68 (18) | |
| VASP-PRI (%)§ | 300 mg (24 h) | 91 (12) | 78 (12) | 68 (16) | 73 (12) |
| 600 mg (24 h) | 85 (14) | 56 (26) | 48 (20) | 51 (20) | |
| 75 mg (Day 5) | 83 (13) | 50 (16) | 39 (14) | 40 (9) | |
| 150 mg (Day 5) | 61 (18) | 29 (11) | 24 (10) | 20 (10) |
Values are mean (SD).
* Intermediate metabolizers have one but not two nonfunctional alleles.
† Ultrarapid metabolizers have at least one gain-of-function allele.
‡ Inhibition of platelet aggregation with 5 mcM ADP; larger value indicates greater platelet inhibition.
§ Vasodilator-stimulated phosphoprotein – platelet reactivity index; smaller value indicates greater platelet inhibition.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of tumorigenicity when clopidogrel was administered for 78 weeks to mice and 104 weeks to rats at dosages up to 77 mg/kg per day, which afforded plasma exposures >25 times that in humans at the recommended daily dose of 75 mg.
Clopidogrel was not genotoxic in four in vitro tests (Ames test, DNA-repair test in rat hepatocytes, gene mutation assay in Chinese hamster fibroblasts, and metaphase chromosome analysis of human lymphocytes) and in one in vivo test (micronucleus test by oral route in mice).
Clopidogrel was found to have no effect on fertility of male and female rats treated prior to pairing and throughout gestation at oral doses up to 400 mg/kg per day (52 times the recommended human dose on a mg/m² basis).
14. Clinical Studies
14.1 Acute Coronary Syndrome
CURE
The CURE study included 12,562 patients with ACS without ST-elevation (UA or NSTEMI) and presenting within 24 hours of onset of the most recent episode of chest pain or symptoms consistent with ischemia. Patients were required to have either ECG changes compatible with new ischemia (without ST-elevation) or elevated cardiac enzymes or troponin I or T to at least twice the upper limit of normal.
Patients were randomized to receive Plavix (300 mg loading dose followed by 75 mg once daily) or placebo, and were treated for up to one year. Patients also received aspirin (75–325 mg once daily) and other standard therapies such as heparin. The use of GPIIb/IIIa inhibitors was not permitted for three days prior to randomization.
The patient population was largely White (82%) and included 38% women, and 52% age ≥65 years of age. Only about 20% of patients underwent revascularization during the initial hospitalization and few underwent emergent or urgent revascularization.
The number of patients experiencing the primary outcome (CV death, MI, or stroke) was 582 (9.3%) in the Plavix-treated group and 719 (11.4%) in the placebo-treated group, a 20% relative risk reduction (95% CI of 10%–28%; p<0.001) for the Plavix-treated group (see Table 4).
Table 4. Outcome Events in the CURE Primary Analysis:
| Outcome | Plavix (+ aspirin)* | Placebo (+ aspirin)* | Relative Risk Reduction () (95 CI) |
|---|---|---|---|
| (n=6259) | (n=6303) | ||
| Primary outcome (Cardiovascular death, MI, stroke) | 582 (9.3%) | 719 (11.4%) | 20% (10.3, 27.9) p<0.001 |
| All Individual Outcome Events:† | |||
| CV death | 318 (5.1%) | 345 (5.5%) | 7% (-7.7, 20.6) |
| MI | 324 (5.2%) | 419 (6.6%) | 23% (11.0, 33.4) |
| Stroke | 75 (1.2%) | 87 (1.4%) | 14% (-17.7, 36.6) |
* Other standard therapies were used as appropriate.
† The individual components do not represent a breakdown of the primary and coprimary outcomes, but rather the total number of subjects experiencing an event during the course of the study.
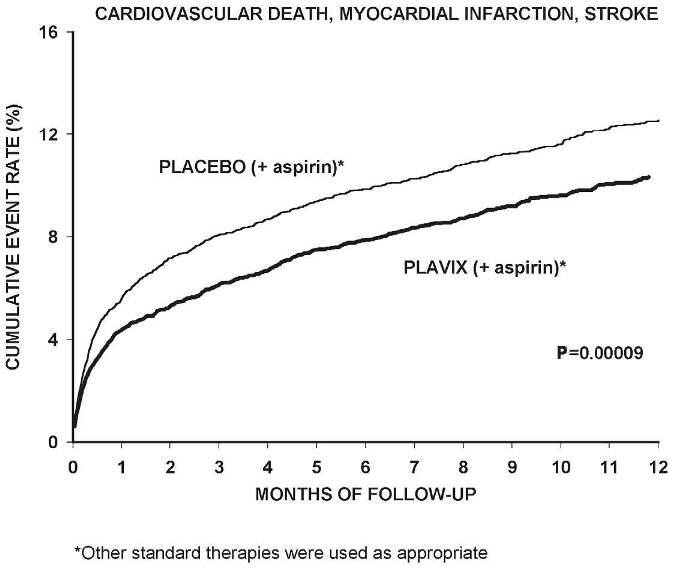
Most of the benefit of Plavix occurred in the first two months, but the difference from placebo was maintained throughout the course of the trial (up to 12 months) (see Figure 2).
Figure 2. Cardiovascular Death, Myocardial Infarction, and Stroke in the CURE Study:
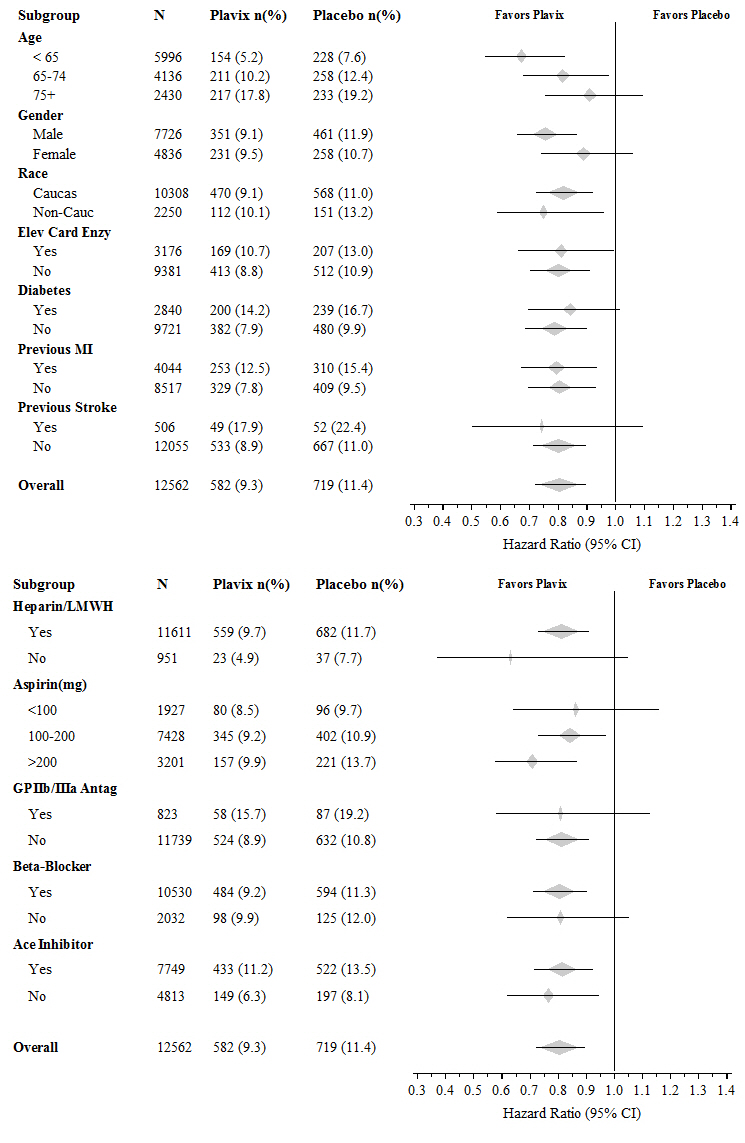
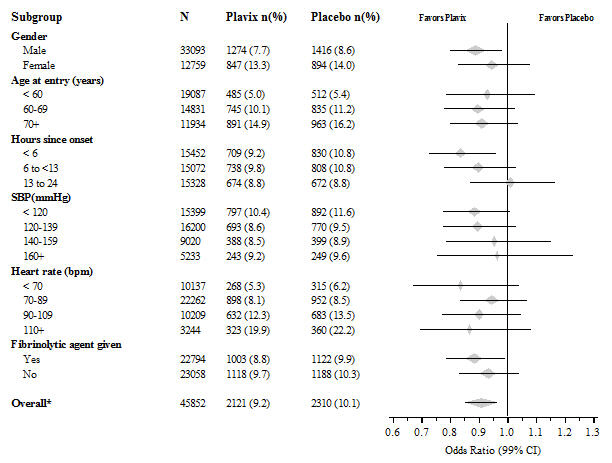
The effect of Plavix did not differ significantly in various subgroups, as shown in Figure 3. The benefits associated with Plavix were independent of the use of other acute and long-term cardiovascular therapies, including heparin/LMWH, intravenous glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors, lipid-lowering drugs, beta-blockers, and ACE inhibitors. The efficacy of Plavix was observed independently of the dose of aspirin (75–325 mg once daily). The use of oral anticoagulants, nonstudy antiplatelet drugs, and chronic NSAIDs was not allowed in CURE.
Figure 3. Hazard Ratio for Patient Baseline Characteristics and On-Study Concomitant Medications/Interventions for the CURE Study:
The use of Plavix in CURE was associated with a decrease in the use of thrombolytic therapy (71 patients [1.1%] in the Plavix group, 126 patients [2.0%] in the placebo group; relative risk reduction of 43%), and GPIIb/IIIa inhibitors (369 patients [5.9%] in the Plavix group, 454 patients [7.2%] in the placebo group, relative risk reduction of 18%). The use of Plavix in CURE did not affect the number of patients treated with CABG or PCI (with or without stenting) (2253 patients [36.0%] in the Plavix group, 2324 patients [36.9%] in the placebo group; relative risk reduction of 4.0%).
COMMIT
In patients with STEMI, the safety and efficacy of Plavix were evaluated in the randomized, placebo-controlled, double-blind study, COMMIT. COMMIT included 45,852 patients presenting within 24 hours of the onset of the symptoms of myocardial infarction with supporting ECG abnormalities (i.e, ST-elevation, ST-depression or left bundle-branch block). Patients were randomized to receive Plavix (75 mg once daily) or placebo, in combination with aspirin (162 mg per day), for 28 days or until hospital discharge, whichever came first.
The primary endpoints were death from any cause and the first occurrence of re-infarction, stroke or death.
The patient population was 28% women and 58% age ≥60 years (26% age ≥70 years). Fifty-five percent (55%) of patients received thrombolytics and only 3% underwent PCI.
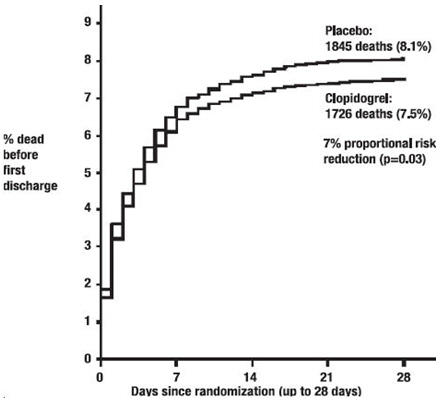
As shown in Table 5 and Figure 4 and Figure 5 below, Plavix significantly reduced the relative risk of death from any cause by 7% (p=0.029), and the relative risk of the combination of re-infarction, stroke or death by 9% (p=0.002).
Table 5. Outcome Events in COMMIT:
| Event | Plavix (+ aspirin) (N=22961) | Placebo (+ aspirin) (N=22891) | Odds ratio (95% CI) | p-value |
|---|---|---|---|---|
| Composite endpoint: Death, MI, or Stroke* | 2121 (9.2%) | 2310 (10.1%) | 0.91 (0.86, 0.97) | 0.002 |
| Death | 1726 (7.5%) | 1845 (8.1%) | 0.93 (0.87, 0.99) | 0.029 |
| Nonfatal MI† | 270 (1.2%) | 330 (1.4%) | 0.81 (0.69, 0.95) | 0.011 |
| Nonfatal Stroke† | 127 (0.6%) | 142 (0.6%) | 0.89 (0.70, 1.13) | 0.33 |
* 9 patients (2 clopidogrel and 7 placebo) suffered both a nonfatal stroke and a nonfatal MI.
† Nonfatal MI and nonfatal stroke exclude patients who died (of any cause).
Figure 4. Cumulative Event Rates for Death in the COMMIT Study*:
Figure 5. Cumulative Event Rates for the Combined Endpoint Re-Infarction, Stroke or Death in the COMMIT Study*:
The effect of Plavix did not differ significantly in various prespecified subgroups as shown in Figure 6. The effect was also similar in non-prespecified subgroups including those based on infarct location, Killip class or prior MI history. Such subgroup analyses should be interpreted cautiously.
Figure 6. Effects of Adding Plavix to Aspirin on the Combined Primary Endpoint across Baseline and Concomitant Medication Subgroups for the COMMIT Study:
* CI is 95% for Overall row only.
14.2 Recent Myocardial Infarction, Recent Stroke, or Established Peripheral Arterial Disease
CAPRIE
The CAPRIE trial was a 19,185-patient, 304-center, international, randomized, double-blind, parallel-group study comparing Plavix (75 mg daily) to aspirin (325 mg daily). To be eligible to enroll, patients had to have: 1) recent history of myocardial infarction (within 35 days); 2) recent histories of ischemic stroke (within 6 months) with at least a week of residual neurological signs; and/or 3) established peripheral arterial disease (PAD). Patients received randomized treatment for an average of 1.6 years (maximum of 3 years).
The trial’s primary outcome was the time to first occurrence of new ischemic stroke (fatal or not), new myocardial infarction (fatal or not), or other vascular death. Deaths not easily attributable to nonvascular causes were all classified as vascular.
Table 6. Outcome Events in the CAPRIE Primary Analysis:
| Patients | Plavix n=9,599 | Aspirin n=9,586 |
|---|---|---|
| Ischemic stroke (fatal or not) | 438 (4.6%) | 461 (4.8%) |
| MI (fatal or not) | 275 (2.9%) | 333 (3.5%) |
| Other vascular death | 226 (2.4%) | 226 (2.4%) |
| Total | 939 (9.8%) | 1020 (10.6%) |
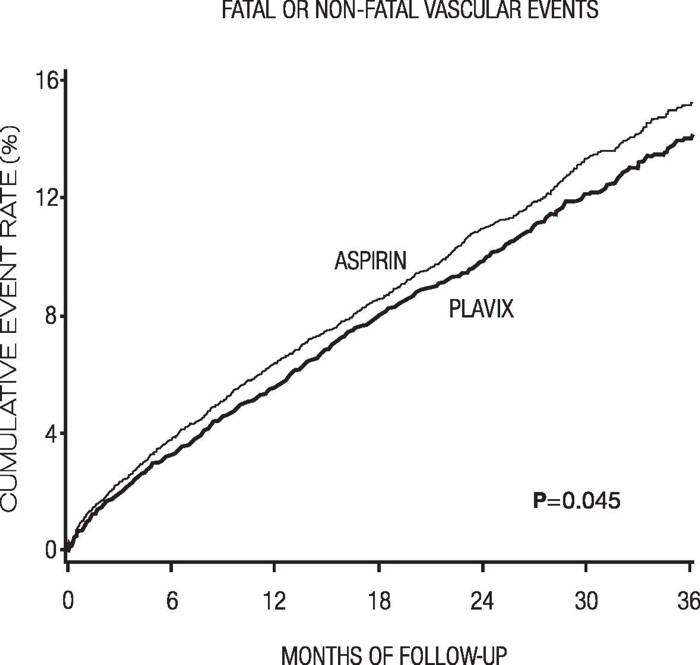
As shown in Table 6, Plavix was associated with a lower incidence of outcome events, primarily MI. The overall relative risk reduction (9.8% vs 10.6%) was 8.7%, p=0.045. Similar results were obtained when all-cause mortality and all-cause strokes were counted instead of vascular mortality and ischemic strokes (risk reduction 6.9%). In patients who survived an on-study stroke or myocardial infarction, the incidence of subsequent events was lower in the Plavix group.
The curves showing the overall event rate are shown in Figure 7. The event curves separated early and continued to diverge over the 3-year follow-up period.
Figure 7. Fatal or Nonfatal Vascular Events in the CAPRIE Study:
The statistical significance favoring Plavix over aspirin was marginal (p=0.045). However, because aspirin is itself effective in reducing cardiovascular events in patients with recent myocardial infarction or stroke, the effect of Plavix is substantial.
The CAPRIE trial enrolled a population that had recent MI, recent stroke, or PAD. The efficacy of Plavix relative to aspirin was heterogeneous across these subgroups (p=0.043) (see Figure 8). Nonetheless, this difference may be a chance occurrence because the CAPRIE trial was not designed to evaluate the relative benefit of Plavix over aspirin in the individual patient subgroups. The benefit was most apparent in patients who were enrolled because of peripheral arterial disease and less apparent in stroke patients. In patients who were enrolled in the trial on the sole basis of a recent myocardial infarction, Plavix was not numerically superior to aspirin.
Figure 8. Hazard Ratio and 95% CI by Baseline Subgroups in the CAPRIE Study:
14.3 No Demonstrated Benefit of Plavix plus Aspirin in Patients with Multiple Risk Factors or Established Vascular Disease
CHARISMA
The CHARISMA trial was a 15,603 subject, randomized, double-blind, parallel group study comparing Plavix (75 mg daily) to placebo for prevention of ischemic events in patients with vascular disease or multiple risk factors for atherosclerosis. All subjects were treated with aspirin 75–162 mg daily. The mean duration of treatment was 23 months. The study failed to demonstrate a reduction in the occurrence of the primary endpoint, a composite of CV death, MI, or stroke. A total of 534 (6.9%) patients in the Plavix group versus 573 (7.4%) patients in the placebo group experienced a primary outcome event (p=0.22). Bleeding of all severities was more common in the subjects randomized to Plavix.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.