QALSODY Solution for injection Ref.[107302] Active ingredients: Tofersen
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
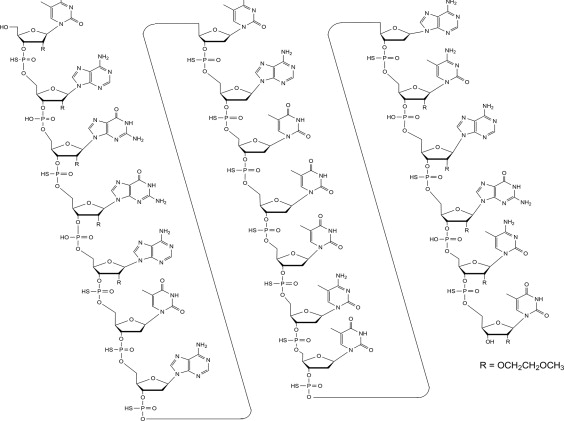
Tofersen, an antisense oligonucleotide, is a 20-base residue (20-mer) 5-10-5 MOE gapmer mixed backbone oligonucleotide. Of the nineteen internucleotide linkages, fifteen are 3′-O to 5′-O phosphorothioate diesters, and four are 3′-O to 5′-O phosphate diesters. Ten of the twenty sugar residues are 2-deoxy-D-ribose and the remainder are 2′-O-(2-methoxyethyl)-D-ribose (MOE). The residues are arranged so that there are five MOE nucleosides at the 5′ and 3′-ends of the molecule flanking a gap of ten 2′-deoxynucleosides. The cytosine and uridine bases are methylated at the 5-position. The structural formula is:
Figure 1. Structural Formula for Tofersen:
The molecular formula is C230H317N72O123P19S15 and the molecular weight is 7127.86 atomic mass units (amu).
QALSODY is supplied as a sterile, preservative-free, clear, and colorless to slightly yellow solution in a Type I glass vial to be administered by intrathecal administration. Each vial of drug product contains a single dose of 100 mg tofersen at a concentration of 6.7 mg/mL in a formulation containing 0.21 mg/mL calcium chloride dihydrate, 0.11 mg/mL dibasic sodium phosphate, 0.16 mg/mL magnesium chloride hexahydrate, 0.03 mg/mL monobasic sodium phosphate, 0.22 mg/mL potassium chloride, 8.77 mg/mL sodium chloride, and water for injection. The pH of QALSODY is approximately 7.2 (range 6.7 to 7.7).
| Dosage Forms and Strengths |
|---|
|
Injection: 100 mg/15 mL (6.7 mg/mL) as a clear and colorless to slightly yellow solution in a single-dose vial. |
| How Supplied |
|---|
|
QALSODY injection is a sterile, clear and colorless to slightly yellow solution supplied as 100 mg/15 mL (6.7 mg/mL) solution in a single-dose glass vial free of preservatives. The NDC is 64406-109-01. Manufactured by: Biogen MA Inc., Cambridge, MA 02142 |
Drugs
| Drug | Countries | |
|---|---|---|
| QALSODY | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.