RAYVOW Film-coated tablet Ref.[112844] Active ingredients: Lasmiditan
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Eli Lilly Nederland B.V., Papendorpseweg 83, 3528 BJ Utrecht, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Analgesics, antimigraine preparations
ATC code: N02CC08
Mechanism of action
Lasmiditan is a high affinity, centrally-penetrant, 5-hydroxytriptamine 1F (5-HT1F) receptor agonist. The precise mechanism of action is unknown, however, the therapeutic effects of lasmiditan in the treatment of migraine presumably involve agonistic effects at the 5-HT1F receptor, a decrease of neuropeptide release and an inhibition of pain pathways, including the trigeminal nerve.
Pharmacodynamic effects
In in vitro binding studies, lasmiditan showed a >440-fold selectivity for the 5-HT1F receptor versus the 5-HT1B and 5-HT1D receptors. Lasmiditan does not constrict, ex-vivo human coronary arteries, ex-vivo human internal mammary arteries, or ex-vivo human middle meningeal arteries, likely due to its low affinity at the vasoconstrictive 5-HT1B receptor.
Cardiac electrophysiology
In a thorough QT study, lasmiditan was associated with a heart rate decrease of 6 bpm when compared to placebo, and administration of a supra-therapeutic dose of 400 mg suggested a prolongation of the QTc in females. Subgroup analyses suggested gender-related differences, since a more pronounced QTc prolongation was observed in the female subset. However, as the maximum recommended dose is limited to 200 mg, no clinically relevant effect is expected.
Clinical efficacy and safety
The efficacy and safety of lasmiditan has been studied in three phase 3, randomized, placebo-controlled, double-blind studies in adult patients (N=5910). The studies enrolled patients aged 18 and older with 3-8 migraine attacks per month, and at least moderately disabling migraine (Migraine Disability Assessment (MIDAS) score ≥11).
Single attack studies
The population enrolled in the single attack studies (SAMURAI and SPARTAN) was predominantly female (84%) with a mean age of 42.3 years. Patients had an average of 5.2 migraine attacks per month in the 3 months prior to enrolment and a mean MIDAS total score of 31.7. SAMURAI, but not SPARTAN, excluded patients with known coronary artery disease, clinically significant arrhythmia, or uncontrolled hypertension. 78.3% of patients had ≥1 cardiovascular risk factor, including age >40 (54.2%), low HDL-cholesterol (31.1%), high blood pressure/hypertension (21.3%), current smoker (14.3%), high total cholesterol (10.9%), and history of diabetes (5.9%), in addition to migraine. 21.7% of patients were prescribed preventive medicinal products for migraine, and 37% had taken a triptan within 3 months of entering the study. The most bothersome symptom (MBS) was photophobia (50.3%), followed by nausea (22.2%), and phonophobia (20.6%). In these studies, a second dose of study drug or other medicinal product was allowed 2 to 24 hours after the initial treatment for persistent or recurrent migraine.
The primary and key secondary endpoints in both studies were the proportion of patients free from pain, and the proportion of patients free from their MBS, compared to placebo at 2 hours after treatment.
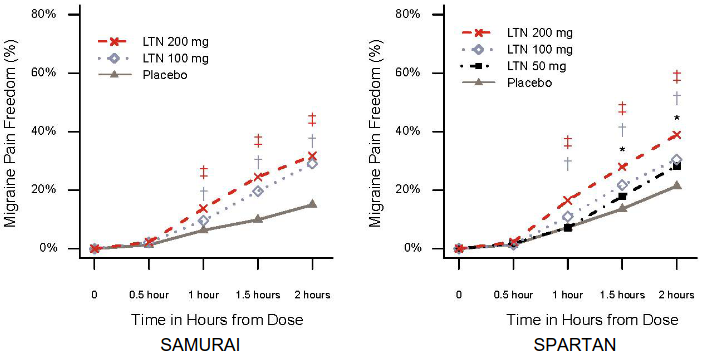
Both studies met the primary and key secondary endpoints. All doses of lasmiditan demonstrated statistically significant and clinically meaningful improvement in the percentage of patients achieving pain freedom, MBS freedom, and pain relief (defined as a reduction in pain severity from moderate or severe at baseline to mild or none or from mild to none) 2 hours after treatment (see Table 2). The timing of onset of pain freedom is demonstrated in Figure 1; onset of pain relief followed the same pattern as pain freedom at 50 mg and 100 mg, with additional separation from placebo seen at the earlier time of 30 mins for the 200 mg dose (17.7% for 200 mg vs 11.6% for placebo, p=0.004 in SAMURAI, 18.6% for 200 mg vs 14.7% for placebo, p=0.014 in SPARTAN).
Table 2. SAMURAI and SPARTAN: Summary of efficacy data:
| SAMURAI | SPARTAN | ||||||
|---|---|---|---|---|---|---|---|
| lasmiditan | Placebo | lasmiditan | Placebo | ||||
| 100 mg | 200 mg | 50 mg | 100 mg | 200 mg | |||
| Pain free at 2 hours | |||||||
| N | 503 | 518 | 524 | 556 | 532 | 528 | 540 |
| Responders (%) | 28.2 | 32.2 | 15.3 | 28.6 | 31.4 | 38.8 | 21.3 |
| p-value | <0.001 | <0.001 | 0.006 | <0.001 | <0.001 | ||
| MBS free at 2 hours | |||||||
| N | 469 | 481 | 488 | 512 | 500 | 483 | 514 |
| Responders (%) | 40.9 | 40.7 | 29.5 | 40.8 | 44.2 | 48.7 | 33.5 |
| p-value | <0.001 | <0.001 | 0.018 | <0.001 | <0.001 | ||
| Pain relief at 2 hours | |||||||
| N | 562 | 555 | 554 | 598 | 571 | 565 | 576 |
| Responders (%) | 54.1 | 54.6 | 39.2 | 55.5 | 59.7 | 60.7 | 44.9 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
Figure 1. Percentage of patients achieving migraine pain freedom within 2 hours in SAMURAI and SPARTAN:
‡ Statistical significance for 200 mg LTN vs placebo; † Statistical significance for 100 mg LTN vs placebo; * Statistical significance for 50 mg LTN vs placebo
Abbreviations: LTN = lasmiditan
Consistency of effect study
In a study assessing the consistency of effect, patients were treated with lasmiditan 100 mg, 200 mg, or control for 4 migraine attacks (CENTURION). In the control group, patients received a single dose of lasmiditan 50 mg to treat either their third or fourth attack and placebo for the remaining attacks. The population enrolled was predominantly female (84%) with a mean age of 41.4 years. Patients had an average of 4.9 migraine attacks per month in the 3 months prior to enrolment and a mean MIDAS total score of 31.9. The study did not exclude patients with cardiovascular diseases, and 58.5% of patients had ≥1 cardiovascular risk factor, including age >40 (52.8%), high total cholesterol (10.8%), high blood pressure/hypertension (16.9%), and history of diabetes (3.1%), in addition to migraine. 28.8% of patients were currently prescribed preventive medicinal products for migraine, and 65.0% had previously taken a triptan. The MBS was photophobia (39.7%), followed by nausea (31.9%), and phonophobia (19.3%).
The co-primary endpoints were the proportion of patients at 2 hours post dose that were free from pain after the first attack, and those that were free from pain in at least 2 out of 3 attacks, compared to placebo.
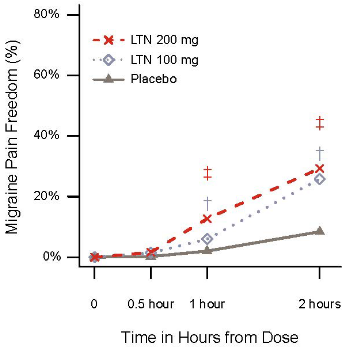
The study met its primary and all key secondary endpoints. Both doses of 100 mg and 200 mg of lasmiditan demonstrated statistically significant and clinically meaningful improvement in the percentage of patients achieving pain freedom, pain relief (a reduction in pain severity from moderate or severe at baseline to mild or none or from mild to none), MBS freedom, 2 hours after treatment, and sustained pain freedom after 24 hours (see Table 3). The timing of onset of pain freedom is demonstrated in Figure 2. Pain relief followed the same pattern as pain freedom at 50 mg and 100 mg, and was observed at the earlier time of 30 minutes with the 200 mg dose (22.4% for 200 mg vs 14.0% for placebo, p=0.001).
Both doses demonstrated consistency of effect with a statistically significant and clinically meaningful improvement in the percentage of patients achieving pain freedom and pain relief in at least 2 out of 3 attacks (see Table 3).
Table 3. CENTURION: Summary of efficacy data:
| lasmiditan | Placebo | ||
|---|---|---|---|
| 100 mg | 200 mg | ||
| Single attack endpoints (ITT) | N=419 | N=434 | N=443 |
| Pain freedom at 2 hours post-dose during first attack | |||
| Responders (%) | 25.8 | 29.3 | 8.4 |
| p-value versus placebo | <0.001 | <0.001 | |
| Pain relief at 2 hours post-dose during first attack | |||
| Responders (%) | 65.4 | 65.2 | 41.3 |
| p-value versus placebo | <0.001 | <0.001 | |
| Sustained pain freedom up to 24 hours post-dose during first attack | |||
| Responders (%) | 13.6 | 17.3 | 4.3 |
| p-value versus placebo | <0.001 | <0.001 | |
| MBS freedom at 2 hours post-dose during first attack | N=376 | N=395 | N=396 |
| Responders (%) | 40.4 | 39.0 | 28.0 |
| p-value versus placebo | <0.001 | 0.001 | |
| Consistency endpoints (ITT Consistency) | |||
| Pain freedom at 2 hours post-dose in at least 2 out of 3 attacks | N=340 | N=336 | N=373 |
| Responders (%) | 14.4 | 24.4 | 4.3 |
| p-value versus placebo | <0.001 | <0.001 | |
| Pain relief at 2 hours post-dose in at least 2 out of 3 attacks | N=332 | N=333 | N=320 |
| Responders (%) | 62.3 | 66.7 | 36.9 |
| p-value versus placebo | <0.001 | <0.001 | |
Abbreviations: ITT = intent to treat
Figure 2. Percentage of patients achieving migraine pain freedom within 2 hours in CENTURION:
‡ Statistical significance for 200 mg LTN vs placebo; † Statistical significance for 100 mg LTN vs placebo
Abbreviations: LTN = lasmiditan
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with RAYVOW in one or more subsets of the paediatric population in the treatment of migraine headaches (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
Following oral administration, lasmiditan is rapidly absorbed with a median tmax of 1.8 hours. In patients with migraine, the pharmacokinetics of lasmiditan was not different during a migraine attack versus during the interictal period. Over the clinical dose range of 50 to 200 mg, the absolute bioavailability is predicted to be 50% to 58% based on results from the population PK analysis. Coadministration of lasmiditan with a high-fat meal increased the mean lasmiditan Cmax and AUC values by 22% and 19%, respectively, and delayed the median tmax by 1 hour. This difference in exposure is not expected to be clinically meaningful. Lasmiditan was administered without regard to food in clinical efficacy studies.
Distribution
The human plasma protein binding of lasmiditan is approximately 55% to 60% and independent of concentration between 15 and 500 ng/mL. The estimated mean volume of distribution was 304 L.
Biotransformation
Lasmiditan undergoes hepatic and extrahepatic metabolism primarily by non-CYP enzymes, with ketone reduction to S-M8 as the major pathway. The following enzymes were not involved in metabolism of lasmiditan: MAO-A, MAO-B, flavin monooxygenase 3, CYP450 reductase, xanthine oxidase, alcohol dehydrogenase, aldehyde dehydrogenase, and aldo-keto reductases.
Lasmiditan is also oxidized on the piperidine ring to M7. Relative to lasmiditan, the metabolites are pharmacologically inactive. Lasmiditan is a substrate of P-gp in vitro.
Lasmiditan and its major metabolites are in vitro inducers of CYP enzymes. Lasmiditan inhibits CYP2D6 in vitro. Lasmiditan and its major metabolite are not inhibitors of MAO-A. Lasmiditan inhibits P-gp, BCRP, and OCT1 efflux transporters in vitro. Lasmiditan inhibits OCT2, MATE1, and MATE2-K renal transporters in vitro.
A clinical drug interaction study indicates that lasmiditan is a weak inhibitor of P-gp (see section 4.5).
Elimination
Lasmiditan was eliminated with a geometric mean t½ value of approximately 5.7 hours. No accumulation of lasmiditan was observed with daily dosing. The estimated mean total body clearance was 66.2 L/h. Lasmiditan generally exhibits linear PK over the clinical dose range of 50 to 200 mg. Lasmiditan is primarily eliminated via metabolism. Renal excretion is a minor route of lasmiditan clearance with approximately 3% of the dose recovered as unchanged lasmiditan in urine. Metabolite S-M8 represented approximately 66% of the dose in urine, with the majority of recovery within 48 hours post-dose.
Special populations
Age, gender, race, ethnicity and body weight
Age, gender, race, ethnicity, and body weight did not have a significant effect on the exposure in a population pharmacokinetic analysis of lasmiditan. In a study, gender had an effect on PK of lasmiditan with higher Cmax (~20-30%) and AUC (~30%) in women compared to men, irrespective whether lasmiditan was administered in fed or fasted conditions. No dose adjustment is necessary based on age, gender, race, ethnicity or body weight.
Renal impairment
Administration of lasmiditan to subjects with severe renal impairment (eGFR <30 mL/min/1.73 m²) demonstrated 18% greater exposure in AUC(0-∞) and 13% higher Cmax, compared to subjects with normal renal function. This difference in exposure is not expected to be clinically significant. No dose adjustment is necessary in patients with mild, moderate, or severe renal impairment.
Hepatic impairment
In subjects with mild and moderate hepatic impairment (Child-Pugh Class A and B, respectively) lasmiditan exposure was 11% and 35%, respectively, higher [AUC(0-∞)] than that in subjects with normal hepatic function. The Cmax were higher by 19% and 33%, respectively, for subjects with mild and moderate hepatic impairment. This difference in exposure is not expected to be clinically significant. No dose adjustment is necessary in patients with mild or moderate hepatic impairment. The use of lasmiditan has not been studied in subjects with severe hepatic impairment and therefore is not recommended for this population.
5.3. Preclinical safety data
Carcinogenicity was assessed in a two-year rat study and a six-month transgenic mouse study. In rats, an increase in pituitary tumour-related deaths in male rats was seen. The relevance of these findings in terms of human risk is unknown. No evidence of carcinogenicity was seen in mice.
Lasmiditan was not genotoxic based on results from the Ames assay in bacteria, a chromosome aberration study in Chinese hamster ovary cells, and micronucleus tests in mice.
Developmental and reproductive toxicity
In studies with rats, there were no effects on male or female fertility.
In embryofoetal development studies with rats and rabbits, decreased foetal body weights and skeletal variations occurred; in rabbits, there was a slight increase in post-implantation loss (embryofoetal mortality), and foetal cardiovascular defects (malformations) occurred at a low incidence. Exposure at the no-observed-adverse effect doses of 175 mg/kg/day (rats) and 75 mg/kg/day (rabbits) were approximately 37 and 1.5-fold, respectively, higher than in humans at 200 mg.
In a rat pre- and postnatal study, prolonged gestation and parturition and an increased number of stillborn pups and frequency of postnatal death occurred at the highest dose tested of 225 mg/kg/day. At this high exposure, a decrease in F1 pup mean body weights observed during the preweaning phase in both genders was maintained throughout the F1 maturation phase with no recovery. Exposure at the no-observed effect dose of 150 mg/kg/day was estimated to be >19 fold higher than that in humans at 200 mg.
All effects occurred at maternally toxic exposures which exceeded human exposure at a clinical dose of 200 mg.
Studies in animals have shown that lasmiditan and/or its metabolites were excreted into the milk of lactating rats.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.