REZDIFFRA Tablet Ref.[108866] Active ingredients: Resmetirom
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
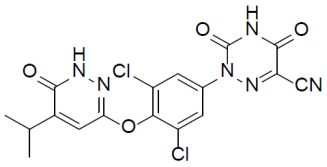
REZDIFFRA (resmetirom) tablets contain resmetirom, a thyroid hormone receptor-beta agonist. The chemical name for REZDIFFRA is 2-[3,5-Dichloro-4-((6-oxo-5-(propan-2-yl)-1,6-dihydropyridazin-3-yl)oxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile. The molecular formula is C17H12Cl2N6O4 and the molecular weight is 435.22. The chemical structure is:
Resmetirom has low aqueous solubility below pH 6 and higher solubility above pH 7 (0.44 mg/mL at pH 7.04).
REZDIFFRA tablets are supplied in 60 mg, 80 mg, and 100 mg strengths for oral administration. Each tablet contains the active ingredient, resmetirom, and the following USP/NF excipients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, mannitol, and microcrystalline cellulose. REZDIFFRA tablets are film-coated with an Opadry coating comprised of polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, red iron oxide (100 mg tablets), yellow iron oxide (80 mg and 100 mg tablets).
| Dosage Forms and Strengths |
|---|
|
REZDIFFRA Tablets:
|
| How Supplied |
|---|
|
REZDIFFRA (resmetirom) tablets are packaged in white high-density polyethylene bottles closed witha child-resistant closure containing an induction seal. 60 mg Tablets: white oval-shaped film-coated tablets, debossed “P60” on one side and plain on theother side.
80 mg Tablets: yellow, oval-shaped, film-coated tablets, debossed with “P80” on one side and plainon the other side.
100 mg Tablets: beige to pink, oval-shaped, film-coated tablets, debossed with"P100" on one side and plain on the other side.
Manufactured by: UPM Pharmaceuticals (Bristol, TN) Manufactured for: Madrigal Pharmaceuticals, West Conshohocken, PA |
Drugs
| Drug | Countries | |
|---|---|---|
| REZDIFFRA | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.