RISVAN Suspension fon injection Ref.[109545] Active ingredients: Risperidone
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
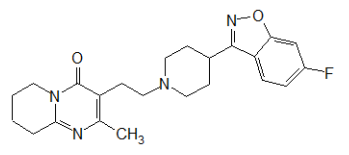
RISVAN contains risperidone, an atypical antipsychotic. Risperidone belongs to the chemical class of benzisoxazole derivatives. The chemical designation 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl) piperidin-1-yl] ethyl]-2-methyl-6,7,8,9- tetrahydropyrido[1,2-a] pyrimidin-4-one. Its molecular formula is C23H27FN4O2 and its molecular weight is 410.5 g/mol.
The structural formula is:
Risperidone is a white to off-white powder. It is practically insoluble in water and soluble in methanol and 0.1 N HCl.
RISVAN is available as a sterile two-syringe mixing system; a solvent syringe prefilled with the solvent dimethyl sulfoxide, a transparent and colorless solution. The powder syringe is prefilled with risperidone and poly (lactide-coglycolide) acid co-polymer. The powder is white to white-yellowish in color.
RISVAN is available as an extended-release injectable suspension, for intramuscular use, in the following strengths of risperidone: 75 mg and 100 mg.
Table 5. RISVAN Constituted Product Delivered Mass:
| Component | RISVAN 75 mg | RISVAN 100 mg |
|---|---|---|
| Risperidone | 75 mg | 100 mg |
| PLGA | 150 mg | 200 mg |
| Dimethylsulfoxide | 350 mg | 466.7 mg |
PLGA Poly (D,L-lactide-co-glycolide) 50:50
| Dosage Forms and Strengths |
|---|
|
RISVAN (risperidone) for extended-release suspension is available in strengths of 75 mg and 100 mg. Each strength is provided as a kit which includes: one pre-filled syringe containing a white to white-yellowish powder in a sealed pouch with a desiccant, one pre-filled syringe containing transparent and colorless solvent in a sealed pouch with a desiccant, one 20-gauge, 2-inch needle for gluteal administration and one 21-gauge, 1 inch needle for deltoid administration. |
| How Supplied |
|---|
|
RISVAN (risperidone) for extended-release injectable suspension is available in dosage strengths of 75 mg and 100 mg that is an off-white to off-white-yellowish, uniform suspension when fully mixed. RISVAN 75 mg is supplied in a single-dose kit, packaged in a carton (NDC 82090-001-01), containing the following:
RISVAN 100 mg is supplied in a single-dose kit, packaged in a carton (NDC 82090-003-01), containing the following:
Manufactured for: Laboratorios Farmacéuticos Rovi S.A. Madrid, Spain Powder syringe manufactured by Laboratorios Farmacéuticos Rovi S.A. Madrid, Spain Solvent syringe manufactured by Rovi Pharma Industrial Services S.A.U. Madrid, Spain |
Drugs
| Drug | Countries | |
|---|---|---|
| RISVAN | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.