RISVAN Suspension fon injection Ref.[109545] Active ingredients: Risperidone
Source: FDA, National Drug Code (US) Revision Year: 2024
1. Indications and Usage
RISVAN is indicated for the treatment of schizophrenia in adults.
2. Dosage and Administration
2.1 General Dosing Information
RISVAN must be administered by a healthcare professional as a deltoid or gluteal intramuscular injection. Do not administer RISVAN by any other route.
For detailed preparation and administration instructions, see Dosage and Administration (2.5).
2.2 Recommended Dosage
Initiate RISVAN at a dose of 75 mg or 100 mg once monthly by deltoid or gluteal intramuscular injection. Do not administer more than one dose (75 mg or 100 mg total) per month.
For patients who have never taken risperidone, establish tolerability with oral risperidone prior to initiating RISVAN.
For patients switching from oral risperidone:
- 3 mg of oral risperidone per day, administer a 75 mg RISVAN dose one day after the last oral risperidone dose.
- 4 mg of oral risperidone per day, administer a 100 mg RISVAN dose one day after the last oral risperidone dose.
Patients who are on stable oral risperidone doses lower than 3 mg per day or higher than 4 mg per day may not be candidates for RISVAN [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
Neither a loading dose nor supplemental oral risperidone is recommended. Administer subsequent RISVAN doses once monthly as a deltoid or gluteal intramuscular injection.
When a dose of RISVAN is missed, administer the next RISVAN dose as soon as possible. Do not administer more than one RISVAN dose per month.
2.3 Dosage Recommendations for Patients with Renal or Hepatic Impairment
Prior to initiating treatment with RISVAN in patients with renal or hepatic impairment, titrate with oral risperidone to atleast 3 mg daily. Following oral titration, and based on clinical response and tolerability, the recommended dosage of RISVAN is 75 mg once monthly [see Use in Specific Populations (8.6, 8.7) and Clinical Pharmacology (12.3)].
2.4 Dosage Modifications for Concomitant Use with Strong CYP2D6 Inhibitors and Strong CYP3A4 Inducers
Co-administration with Strong CYP2D6 Inhibitors
Between 2 to 4 weeks prior to initiating a strong CYP2D6 inhibitor, switch patients (if applicable) to the lowest RISVAN dosage (75 mg once monthly) to adjust for the expected increase in plasma concentrations of risperidone [see Drug Interactions (7.1)].
When a strong CYP2D6 inhibitor is initiated in patients receiving RISVAN 75 mg, continue treatment with 75 mg unless clinical judgment necessitates interruption of RISVAN treatment [see Drug Interactions (7.1)].
Co-administration with Strong CYP3A4 Inducers
With concomitant use of RISVAN 75 mg and strong CYP3A4 inducers, increase the RISVAN dosage to 100 mg once monthly. In patients receiving RISVAN 100 mg, additional oral risperidone therapy may need to be considered.
Upon discontinuation of a strong CYP3A4 inducer in a patients receiving RISVAN 100 mg once monthly, re-evaluate the dosage of RISVAN or any additional oral risperidone therapy and, if necessary, decrease to adjust for the expected increase in plasma concentration of risperidone.
Upon discontinuation of a strong CYP3A4 inducer in a patient receiving RISVAN 75 mg once monthly, continue treatment with the 75 mg dose unless clinical judgment necessitates interruption of RISVAN treatment [see Drug Interactions (7.1)].
2.5 Preparation and Administration Instructions
- To be prepared and administered by a healthcare professional only.
- Read the instructions for preparation and administration below and consider referring to the separate Healthcare Provider “Instructions for Use” for preparation and administration considerations.
- Label the administration syringe immediately after reconstitution, then administer immediately.
- For deltoid or gluteal intramuscular injection only. Do not inject by any other route. As a universal precaution, always wear gloves.
- Do not substitute any component of the drug kit.
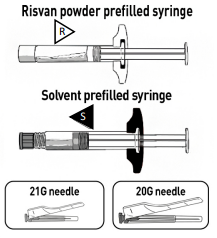
STEP 1 CHECK CONTENTS
Working on a clean surface, open the pouches and discard the desiccant.
Each carton of RISVAN contains:
- One pouch with a RISVAN prefilled syringe with a WHITE plunger rod and WHITE finger flange.
- One pouch with SOLVENT for RISVAN prefilled syringe with a TRANSPARENT plunger rod and a colored finger flange.
- Two administration needles 21G, 1 inch for deltoid (green cap) and a 20G, 2 inch for gluteus (yellow cap).
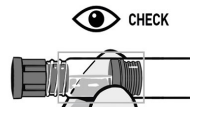
Visually inspect RISVAN for particles and discoloration prior to administration. Discard the kit if any component is damaged.
INSPECT SOLVENT SYRINGE
ENSURE that SOLVENT syringe content flows normally as a liquid. The solvent freezes at 66 ºF. If the solvent is frozen or partially frozen, warm it at room temperature until it flows normally.
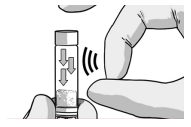
DISLODGE POWDER SYRINGE
TAP the RISVAN syringe to dislodge potential packed powder near the cap.
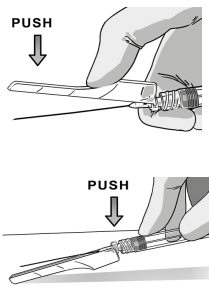
STEP 2 CONNECT THE SYRINGES
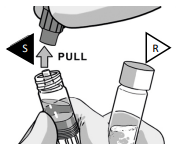
UNCAP SYRINGES IN UPRIGHT POSITION
Hold both syringes in upright position to prevent loss of product.
PULL the cap off the Solvent syringe.
TWIST and PULL the Powder syringe cap off.
Make sure that Powder syringe R is in the upright position to prevent loss of product.
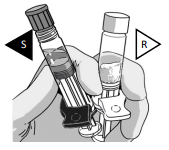
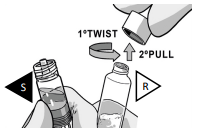
CONNECT THE SYRINGES
Pick the solvent syringe “S” that has the colored finger flange and place it on TOP of the powder syringe “R”, or slightly tilt it vertically.
TWIST the syringes together until you feel a slight resistance.
Make sure that Powder syringe R is in the upright position to prevent loss of product.
STEP 3 MIX THE CONTENTS
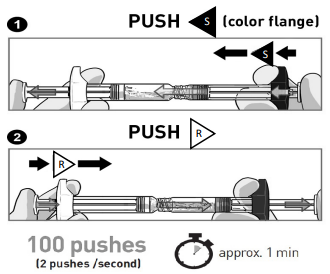
- STOP AND READ THIS SECTION BEFORE STARTING OR THE MEDICATION MAY NOT CORRECTLY RECONSTITUTE.
- PUSH VIGOROUSLY the Solvent content “S” towards the Powder syringe.
- DO NOT WAIT for powder wetting and QUICKLY start mixing contents by pushing the plungers FAST and alternately for 100 pushes (2 pushes within 1 second, approximately 1 minute).
- ENSURE medication is passing between both syringes for proper mixing: medication is viscous and you will need to apply force when pressing on the plunger rods.
Mix for at least 100 pushes by doing alternately 1 followed by 2
Make sure medication is passing between both syringes
When medication is correctly mixed, the appearance will be a uniform suspension off white to yellowish color and thick consistency.
Proceed immediately to prepare the injection for administration.
STEP 4 PREPARE INJECTION SYRINGE
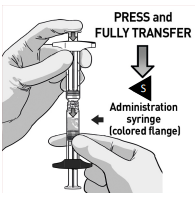
TRANSFER MEDICATION
Place downward pressure on the “R” plunger rod and transfer all the content into the “S” syringe that has the colored flange.
Make sure all the content is transferred.
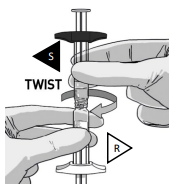
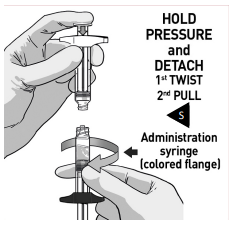
DETACH SYRINGES
Once the medication is fully transferred, separate the two syringes by untwisting.
Proceed immediately to prepare the injection syringe for administration.
The injection must be given within 15 minutes after reconstitution.
ATTACH THE SAFETY NEEDLE
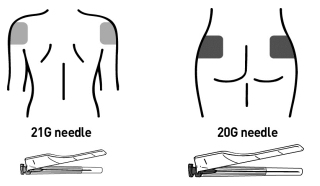
Choose the proper needle:
- Deltoid: 21G, 1 inch for deltoid (green cap).
- Gluteus: 20G, 2 inch for gluteus (yellow cap).
Attach it using a clockwise twisting motion. Do not over-tighten.
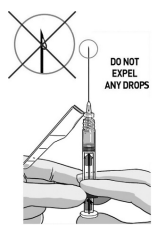
REMOVE EXCESS AIR
Remove needle cover and push out the excess air (only big bubbles) from the syringe barrel.
DO NOT expel any drops of medication
If you see the medication appearing at the needle tip, pull slightly back on the plunger to prevent medication spillage.
STEP 5 ADMINISTER AND DISPOSE
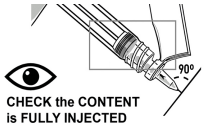
INJECT MEDICATION
Insert the needle fully into the deltoid or gluteal muscle. DO NOT INJECT BY ANY OTHER ROUTE.
- WARNINGS
- THICK MEDICATION, INJECT IT SLOWLY AND STEADILY. MAKE SURE TO FULLY INJECT IT.
- The injection time is longer than usual due to the viscosity of the medication.
- Wait a few seconds before removing the needle.
DISPOSE OF NEEDLE AND SYRINGE.
Cover the needle by pressing on the needle guard using a finger or a flat surface and dispose immediately.
10. Overdosage
Human Experience
Premarketing experience with oral risperidone included eight reports of overdosage with estimated doses ranging from 20 to 300 mg and no fatalities. In general, reported signs and symptoms were those resulting from an exaggeration of the drug’s known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, and extrapyramidal symptoms. One case, involving an estimated overdose of 240 mg, was associated with hyponatremia, hypokalemia, prolonged QT, and widened QRS. Another case, involving an estimated overdose of 36 mg, was associated with a seizure.
Postmarketing experience includes reports of acute oral risperidone overdosage, with estimated doses of up to 360 mg. In general, the most frequently reported signs and symptoms are those resulting from an exaggeration of the drug’s known pharmacological effects, i.e., drowsiness, sedation, tachycardia, hypotension, and extrapyramidal symptoms. Other adverse reactions reported since market introduction related to oral risperidone overdose include prolonged QT interval and convulsions. Torsade de pointes has been reported in association with combined overdose of oral risperidone and paroxetine.
Management of Overdosage
There is no specific antidote to risperidone. Provide supportive care including close medical supervision and monitoring. Treatment should consist of general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdosage. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs.
The pharmacokinetic profile of RISVAN should be considered when treating patients with overdose.
Consider contacting the Poison Help Line (1-800-222-1222) or medical toxicologist for additional overdosage management recommendations.
16.2. Storage and Handling
Store at room temperature 20°C to 25°C (68°F to 77°F) with excursions permitted between 15°C and 30°C (between 59°F and 86°F) in the unopened original packaging.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.