RIVFLOZA Solution for injection Ref.[107412] Active ingredients: Nedosiran
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
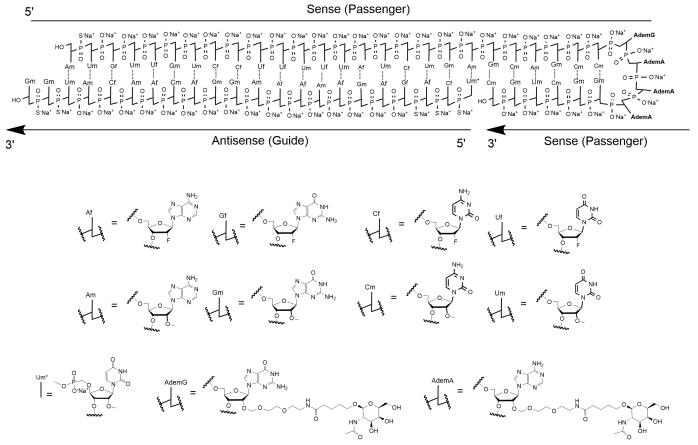
RIVFLOZA injection contains nedosiran, a double-stranded small interfering RNA (siRNA) with four covalently attached N-acetyl-D-galactosamine (GalNAc) residues. Nedosiran targets lactate dehydrogenase A (LDHA) in hepatocytes via GalNAc-mediated delivery.
The structural formula of the nedosiran sodium drug substance is presented below:
The molecular formula of nedosiran sodium is C662H808F19N231O413P57S6Na57 with a molecular weight of 22,238 Da. Nedosiran sodium is freely soluble in water.
RIVFLOZA Pre-filled Syringe is supplied as a clear, sterile, preservative-free, colorless‑to‑yellow solution for subcutaneous injection containing either the equivalent of 160 mg (present as 170 mg nedosiran sodium salt) nedosiran in 1 mL or the equivalent of 128 mg (present as 136 mg nedosiran sodium salt) nedosiran in 0.8 mL of water for injection and sodium hydroxide and/or hydrochloric acid to adjust the pH to ~7.2.
RIVFLOZA vial is supplied as a clear, sterile, preservative-free, colorless-to-yellow solution for subcutaneous injection containing the equivalent of 80 mg (present as 85 mg nedosiran sodium salt) nedosiran in 0.5 mL of water for injection and sodium hydroxide and/or hydrochloric acid to adjust the pH to ~7.2.
| Dosage Forms and Strengths |
|---|
|
RIVFLOZA Injection 160 mg/mL (present as 170 mg nedosiran sodium salt) is a clear, colorless-to-yellow solution available as follows:
|
| How Supplied | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
RIVFLOZA is a clear, sterile, preservative-free, colorless-to-yellow solution available in singledose pre-filled syringes and single-dose vials in cartons containing one unit each. Table 3. RIVFLOZA Presentations:
Manufactured by: Pyramid Laboratories, 3598 Cadillac Ave, Costa Mesa, CA 92626 |
Drugs
| Drug | Countries | |
|---|---|---|
| RIVFLOZA | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.