RIVFLOZA Solution for injection Ref.[107412] Active ingredients: Nedosiran
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
Nedosiran is a double-stranded siRNA, conjugated to GalNAc aminosugar residues. After subcutaneous administration, the GalNAc-conjugated sugars bind to asialoglycoprotein receptors (ASGPR) to deliver nedosiran to hepatocytes.
Nedosiran reduces levels of hepatic lactate dehydrogenase (LDH) via the degradation of LDHA messenger ribonucleic acid (mRNA) in hepatocytes through RNA interference. The reduction of hepatic LDH by nedosiran reduces the production of oxalate by the liver, thereby reducing subsequent oxalate burden.
12.2. Pharmacodynamics
The pharmacodynamic effects of RIVFLOZA were evaluated after single-dose and monthly-dose administration in patients with PH1. Dose-dependent reductions in urinary oxalate were observed in the single-dose range of 1.5 mg/kg to 6.0 mg/kg. With the recommended monthly dose regimen of RIVFLOZA, onset of effect was observed at the first measurement (30 days after the first dose) and the effect persisted with continued monthly dosing [see Clinical Studies (14.1)].
Cardiac Electrophysiology
At the recommended dose, RIVFLOZA does not lead to clinically relevant QT interval prolongation.
12.3. Pharmacokinetics
The pharmacokinetic (PK) properties of RIVFLOZA were evaluated following administration of single and multiple dosages in patients with PH1 or PH2 as summarized in Table 2.
Table 2. Pharmacokinetic Parameters of Nedosiran:
| Nedosiran | |||||
|---|---|---|---|---|---|
| General Information | |||||
| Steady State Exposure | Cmax [Mean (%CV)] | 844 (44) ng/mL | |||
| AUC0-last [Mean (%CV)] | 13600 (36) ng*h/mL | ||||
| Dose Proportionality | Nedosiran exhibited a dose-proportional increase in plasma exposure following single subcutaneous doses from 1.5 to 6.0 mg/kg. Nedosiran exhibited time-independent pharmacokinetics with multiple doses of 160 mg once monthly (body weight ≥50 kg), 128 mg once monthly (body weight <50 kg),or 3.3 mg/kg once monthly in the age range of 6 to 11 years. | ||||
| Accumulation | No accumulation of nedosiran was observed in plasma following repeated monthly dosing. | ||||
| Absorption | |||||
| Tmax [Median (Range)] | 6 (2 to 12) hours | ||||
| Distributiona | |||||
| Estimated Vz/F | 126 L | ||||
| Protein Binding | 85.6% | ||||
| Elimination | |||||
| Half-Life (Mean (%CV)]) | 15 (68) hours | ||||
| Estimated CL/F | 5.7 L/hr | ||||
| Metabolism | |||||
| Primary Pathway | Nedosiran is metabolized by endo- and exonucleases to shorter oligonucleotides. | Excretion | |||
|\2 Primary Pathway|Approximately 27% of the administered nedosiran dose is excreted
unchanged into the urine within 24 hours of dosing.
a Nedosiran distributes primarily to the liver after subcutaneous administration.
Cmax = maximum plasma concentration; AUC0-last = area under the plasma concentration-time curve from time of administration (0) to the last measurable time point (last); Tmax = time to maximum concentration; Vd/F = apparent volume of distribution; CV = coefficient of variation; CL/F = apparent clearance.
Specific Populations
No clinically significant differences in the pharmacokinetics or pharmacodynamics of nedosiran were observed based on age (9 to 73 years old), sex, race/ethnicity, mild-to-moderate renal impairment (eGFR 30 to 89 mL/min/1.73 m²]) [see Use in Specific Populations (8.7)] or mild hepatic impairment as assessed using the National Cancer Institute Organ Dysfunction Working Group criteria (total bilirubin ≤ ULN and AST > ULN; or total bilirubin >1 to 1.5 × ULN and any AST) [see Use in Specific Populations (8.6)].
Pediatrics
At the recommended clinical dose, PK exposure of nedosiran is similar in adult and pediatric patients 9 years of age and older.
Drug Interaction Studies
Concomitant use of pyridoxine (vitamin B6) did not have a significant impact on the PK of nedosiran.
In vitro studies demonstrated that nedosiran was not an inhibitor or inducer of cytochrome P450 (CYP) enzymes and was neither a substrate nor an inhibitor of efflux and uptake transporters.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Long-term studies to assess carcinogenic risk of nedosiran have not been conducted.
Genotoxicity
Nedosiran was not genotoxic in the in vitro bacterial mutagenicity, in vitro micronucleus assays (human peripheral blood lymphocytes) and in vivo bone marrow micronucleus assay in mice.
Fertility
Weekly subcutaneous administration of nedosiran at doses of 500, 1000, or 2000 mg/kg or of a mouse-specific (pharmacologically active) analog at a dose of 10 mg/kg to male mice for 4 weeks prior to and throughout mating, and to female mice for 2 weeks prior to and throughout mating and to gestation day 7 did not affect male or female fertility or early embryonic development.
14. Clinical Studies
14.1 PHYOX2
PHYOX2 was a randomized, double-blind trial comparing RIVFLOZA and placebo in patients aged 6 years or older with PH1 or PH2 and an eGFR ≥30 mL/min/1.73 m² (NCT03847909). Too few PH2 patients were enrolled to evaluate efficacy in the PH2 population. Therefore, RIVFLOZA is only indicated for patients with PH1 [see Indications and Usage (1)]. Unless otherwise noted, data are presented for the complete study population (PH1 and PH2).
Patients received monthly doses of RIVFLOZA (N=23) or placebo (N=12). The RIVFLOZA dose for patients at least 12 years of age weighing at least 50 kg was 160 mg, for patients at least 12 years of age weighing less than 50 kg was 128 mg, and for children 6 to 11 years of age was 3.3 mg/kg (to a maximum of 128 mg).
The median age was 20 years (range 9-46 years), 51% were female, 71% were White, 17% were Asian, 83% had PH1, and 17% had PH2. At baseline, mean 24-hour urinary oxalate excretion, normalized by 1.73 m² BSA in patients less than 18 years of age, was 1547 µmol/24-hour. Mean plasma oxalate was 8.2 µmol/L, 43% of patients had an eGFR ≥90 mL/min/1.73 m², 34% had an eGFR 60 to <90 mL/min/1.73 m², 23% had an eGFR 30 to <60 mL/min/1.73 m², and 60% were taking pyridoxine.
The primary efficacy endpoint was the area under the curve, from Days 90 to 180, of the percent change from baseline in 24-hour urinary oxalate excretion (AUC24-hour Uox). The least-squares (LS) mean AUC24-hour Uox was -3486 (95% CI: -5025, -1947) in the RIVFLOZA group compared to 1490 (95% CI: 781, 3761) in the placebo group, for a between group difference of 4976 (95% CI: 2803, 7149; p<0.0001).
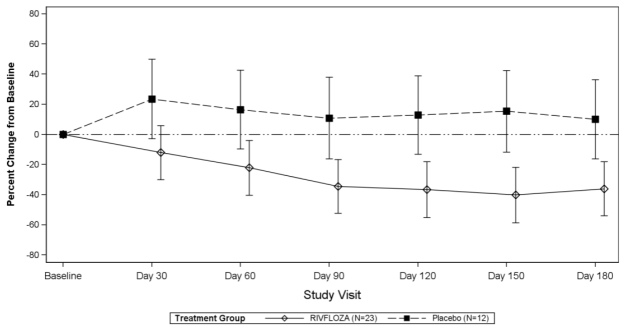
The LS mean percent change from baseline in 24-hour urinary oxalate excretion (corrected for BSA in patients <18 years of age) averaged over Days 90, 120, 150 and 180, was -37% (95% CI: -53%, -21%) in the RIVFLOZA group and 12% (95% CI: -12%, 36%) in the placebo group, for a between group difference of 49% (95% CI: 26%, 72%) [Figure 1]. Among patients with PH1, the between group difference was 56% (95% CI: 33%, 80%).
Figure 1. Mean (95% CI) Percent Change from Baseline in 24-hour Urinary Oxalate in RIVFLOZA and Placebo-Treated Patients in PHYOX2:
After 6 months of treatment in PHYOX2, patients could enroll in an ongoing single-arm extension study, PHYOX3 (NCT04042402), in which all patients were treated with RIVFLOZA. The reduction in urinary oxalate was maintained in the 13 patients with PH1 who received an additional 6 months of treatment in PHYOX3.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.