SELARSDI Solution for injection Ref.[109631] Active ingredients: Ustekinumab
Source: FDA, National Drug Code (US) Revision Year: 2024
1. Indications and Usage
1.1 Plaque Psoriasis (PsO)
SELARSDI is indicated for the treatment of adults and pediatric patients 6 years of age and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
1.2 Psoriatic Arthritis (PsA)
SELARSDI is indicated for the treatment of adults and pediatric patients 6 years of age and older with active psoriatic arthritis.
2. Dosage and Administration
2.1 Recommended Dosage in Plaque Psoriasis
Subcutaneous Adult Dosage Regimen
- For patients weighing 100 kg or less, the recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients weighing more than 100 kg, the recommended dose is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.
In subjects weighing more than 100 kg, 45 mg was also shown to be efficacious. However, 90 mg resulted in greater efficacy in these subjects [see Clinical Studies (14)].
Subcutaneous Pediatric Dosage Regimen
Administer SELARSDI subcutaneously at Weeks 0 and 4, then every 12 weeks thereafter.
The recommended dose of SELARSDI for pediatric patients (6 to 17 years old) with plaque psoriasis based on body weight is shown below (Table 1).
Table 1. Recommended Dose of SELARSDI for Subcutaneous Injection in Pediatric Patients (6 to 17 years old) with Plaque Psoriasis:
| Body Weight of Patient at the Time of Dosing | Recommended Dose |
|---|---|
| 60 kg to 100 kg | 45 mg |
| more than 100 kg | 90 mg |
There is no dosage form for SELARSDI that allows weight-based dosing for pediatric patients below 60 kg.
2.2 Recommended Dosage in Psoriatic Arthritis
Subcutaneous Adult Dosage Regimen
- The recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients with co-existent moderate-to-severe plaque psoriasis weighing more than 100 kg, the recommended dose is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.
Subcutaneous Pediatric Dosage Regimen
Administer SELARSDI subcutaneously at Weeks 0 and 4, then every 12 weeks thereafter.
The recommended dose of SELARSDI for pediatric patients (6 to 17 years old) with psoriatic arthritis, based on body weight, is shown below (Table 2).
Table 2. Recommended Dose of SELARSDI for Subcutaneous Injection in Pediatric Patients (6 to 17 years old) with Psoriatic Arthritis:
| Body Weight of Patient at the Time of Dosing | Recommended Dose |
|---|---|
| 60 kg or more | 45 mg |
| greater than 100 kg with co-existent moderate-to-severe plaque psoriasis | 90 mg |
There is no dosage form for SELARSDI that allows weight-based dosing for pediatric patients below 60 kg.
2.3 General Considerations for Administration
- SELARSDI is intended for use under the guidance and supervision of a healthcare provider. SELARSDI should only be administered to patients who will be closely monitored and have regular follow-up visits with a healthcare provider. The appropriate dose should be determined by a healthcare provider using the patient’s current weight at the time of dosing. In pediatric patients, it is recommended that SELARSDI be administered by a healthcare provider. If a healthcare provider determines that it is appropriate, a patient may self-inject or a caregiver may inject SELARSDI after proper training in subcutaneous injection technique. Instruct patients to follow the directions provided in the Medication Guide [see Medication Guide].
- Not made with natural rubber latex.
- It is recommended that each injection be administered at a different anatomic location (such as upper arms, gluteal regions, thighs, or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, or indurated.
- Prior to administration, visually inspect SELARSDI for particulate matter and discoloration. SELARSDI is a clear and colorless to slightly yellow solution and free of visible particles. Do not use SELARSDI if it is discolored or cloudy, or if other particulate matter is present. SELARSDI does not contain preservatives; therefore, discard any unused product remaining in the syringe.
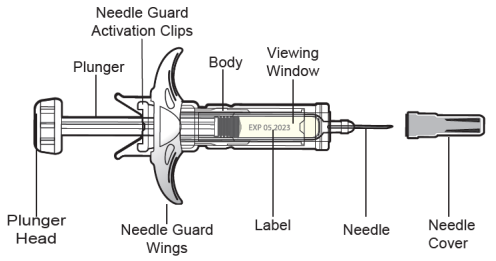
2.4 Instructions for Administration of SELARSDI Prefilled Syringes Equipped with Passive Safety Device
Refer to the diagram below for the provided instructions.
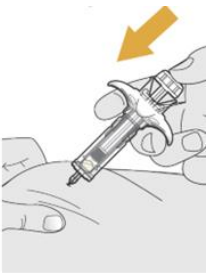
- Hold the SYRINGE BODY and remove the NEEDLE COVER. Do not hold the PLUNGER or PLUNGER HEAD while removing the NEEDLE COVER or the PLUNGER may move. Do not use the prefilled syringe if it is dropped without the NEEDLE COVER in place.
- Inject SELARSDI subcutaneously as recommended [see Dosage and Administration (2.1, 2.2, 2.3)].
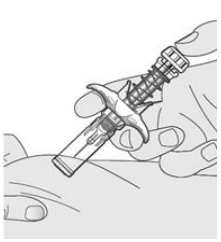
- Inject all of the medication by pushing in the PLUNGER HEAD all the way in until the plunger head is completely between the needle guard activation clips. Injection of the entire prefilled syringe contents is necessary to activate the passive safety device guard.
After injection, maintain the pressure on the PLUNGER HEAD and remove the needle from the skin. Slowly take your thumb off the PLUNGER HEAD. The PLUNGER will move up with your thumb and retract the needle into the needle guard, as shown by the illustration below:
- Used syringes should be placed in a puncture-resistant container.
10. Overdosage
Single doses up to 6 mg/kg intravenously have been administered in clinical trials without dose-limiting toxicity. In case of overdosage, monitor the patient for any signs or symptoms of adverse reactions or effects and institute appropriate symptomatic treatment immediately. Consider contacting the Poison Help line (1-800- 222-1222) or a medical toxicologist for additional overdose management recommendations.
16.2. Storage and Handling
SELARSDI prefilled syringes must be refrigerated at 2°C to 8°C (36°F to 46°F). Keep the product in the original carton to protect from light until the time of use. Do not freeze. Do not shake.
If needed, individual prefilled syringes may be stored at room temperature up to 30°C (86°F) for a maximum single period of up to 30 days in the original carton to protect from light. Record the date when the prefilled syringe is first removed from the refrigerator on the carton in the space provided. Once a syringe has been stored at room temperature, do not return to the refrigerator. Discard the syringe if not used within 30 days at room temperature storage. Do not use SELARSDI after the expiration date on the carton or on the prefilled syringe.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.