SIXMO Implant Ref.[28026] Active ingredients: Buprenorphine
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: L. Molteni & C. dei F.lli Alitti Soc.Es.S.p.A, Strada Statale 67, 50018 Scandicci (Firenze), Italy
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other nervous system drugs, Drugs used in opioid dependence
ATC code: N07BC01
Mechanism of action
Buprenorphine is an opioid partial agonist/antagonist which binds to the μ (mu) and κ (kappa) receptors of the brain. Its activity in opioid maintenance treatment is attributed to its slowly reversible properties at the µ receptors which, over a prolonged period, minimises the need for use of other opioids.
During clinical pharmacologic studies in opioid-dependent patients, buprenorphine shows ceiling effects on a number of PD and safety parameters. It has a relatively wide therapeutic window as a consequence of its partial agonist/antagonist properties, which attenuates suppression of cardiovascular and respiratory function.
Clinical efficacy and safety with Sixmo
The safety and efficacy of Sixmo was investigated in 3 double-blind Phase 3 clinical studies in which a total of 309 patients were treated with Sixmo for up to 6 months (1 implant cycle). Of these 309 patients, 107 patients were treated for an additional 6 months in extension studies (i.e. for 2 implant cycles).
The demonstration of efficacy relies primarily on study PRO-814, a randomized, double-blind and active-controlled Phase 3 study in adult patients who met DSM-IV-TR criteria for opioid dependence and who were clinically stabilised on sublingual buprenorphine. In this study, approximately 75% of patients reported prescription opioids as the primary opioid of abuse, and 21% of patients reported heroin as the primary opioid of abuse. The implant time was 24 weeks. This study enrolled 84 patients in the Sixmo group and 89 patients in the sublingual buprenorphine group, with a median age (range) of 36 (21 to 63) years and 37 (22 to 64) years in the Sixmo and sublingual buprenorphine groups, respectively. In this double-blind and double-dummy study, patients maintained on doses of sublingual buprenorphine of 8 mg/day or less were transferred to 4 Sixmo implants (and daily sublingual placebo), or sublingual buprenorphine 8 mg/day or less (and 4 placebo implants). The primary endpoint was proportion of responders, defined as patients with no more than 2 of 6 months with evidence of illicit opioid use based on a composite of both urine and self-report results. This endpoint was considered to be of clinical relevance in the targeted indication. Sixmo was shown to be noninferior to sublingual buprenorphine, the proportion of responders being 87.6% in the sublingual buprenorphine and 96.4% in the Sixmo group. Furthermore, after establishment of non-inferiority, superiority of Sixmo over sublingual buprenorphine was tested and established (p=0.034). Retention in treatment was high, with 96.4% of Sixmo patients and 94.4% of sublingual buprenorphine patients completing the study.
Two additional randomised, double-blind, placebo-controlled Phase 3 studies provide supportive data on efficacy and PK (Studies PRO-805 and PRO-806). In both studies adult patients with opioid dependence who were new entrants to buprenorphine treatment were treated over 24 weeks with 4 Sixmo or 4 placebo implants. Patients not adequately treated with the 4 implant dose could receive a fifth implant. Study PRO-806 included an open-label comparator arm with sublingual buprenorphine (12 to 16 mg/day). Patients in all groups were allowed to use supplemental sublingual buprenorphine to control potential withdrawal symptoms/cravings according to pre-specified criteria. Patient characteristics in these studies are shown below.
Table 2. Patient characteristics in the studies PRO-805 and PRO-806:
| Study PRO-805 | Study PRO-806 | ||||
|---|---|---|---|---|---|
| Sixmo N=108 | Placebo N=55 | Sixmo N=114 | Placebo N=54 | sublingual buprenorphine N=119 | |
| Median age (range), years | 33 (19-62) | 39 (20-61) | 36 (19-60) | 33 (19-59) | 32 (18-60) |
| Primary opioid of abuse, n (%) | |||||
| Heroin | 69 (63.9%) | 34 (61.8%) | 76 (66.7%) | 28 (51.9%) | 75 (63.0%) |
| Prescription opioids | 39 (36.1%) | 21 (38.2%) | 38 (33.3%) | 26 (48.1%) | 43 (36.1%)* |
* For 1 patient (0.8%) primary opioid of abuse was “other”.
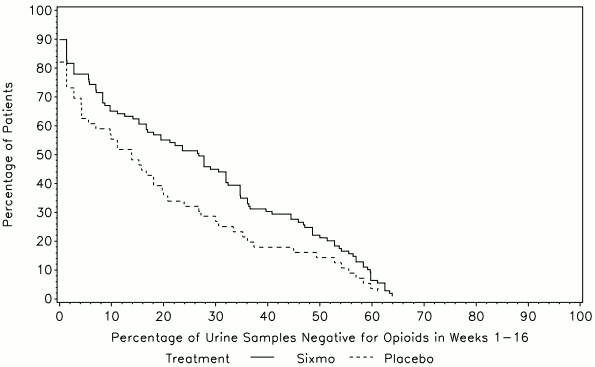
The primary efficacy endpoint in both studies was the cumulative distribution function (CDF) of the percentage of urine samples that were negative for illicit opioids (as evaluated through thrice weekly urine toxicology and patient self-reported opioid use).
In study PRO-805, the primary endpoint was the CDF of the percentage of urine samples that were negative for illicit opioids over weeks 1 to 16, while the CDF over weeks 17 to 24 was evaluated as secondary endpoint.
Table 3. Percentage of Opioid-Negative Urine Samples for Weeks 1 to 16 and Weeks 17 to 24, Study PRO-805 (ITT):
| Percentage of negative results | Sixmo N=108 | Placebo N=55 |
|---|---|---|
| Weeks 1 to 16 | ||
| Mean (SE) | 40.4 (3.15) | 28.3 (3.97) |
| CI of mean | 34.18, 46.68 | 20.33, 36.26 |
| Median (Range) | 40.7 (0, 98) | 20.8 (0, 92) |
| <bWeeks 17 to 24 | ||
| Mean (SE) | 29.0 (3.34) | 10.7 (3.19) |
| CI of mean | 22.41, 35.66 | 4.33, 17.12 |
| Median (Range) | 4.4 (0, 100) | 0.0 (0, 92) |
CI=confidence interval, ITT=intent-to-treat, N=number of subjects, SE=standard error
In the analysis of the CDF (weeks 1 to 16), a statistically significant difference between treatments (p=0.0361) was seen, which was in favour of Sixmo.
Figure 1. Cumulative Distribution Function of the Percentage of Urine Samples Negative for Opioids in Weeks 1-16, Study PRO-805 (ITT):
ITT=intent-to-treat
Buprenorphine was not included in urine toxicology assessments.
Study PRO-806 had two co-primary endpoints, which were the CDF of the percentage of urine samples that were negative for illicit opioids for Weeks 1 to 24 in the Sixmo and placebo groups (coprimary 1), and the CDF of the percentage of urine samples that were negative for illicit opioids for Weeks 1 to 24 in the Sixmo and placebo groups, with imputation based on illicit drug self-report data (co-primary 2).
Table 4. Percentage of Opioid-Negative Urine Samples for Weeks 1 to 24, Study PRO-806 (ITT):
| Percentage of negative results | Sixmo N=114 | Placebo N=54 | Sublingual buprenorphine N=119 |
|---|---|---|---|
| Mean (SE) | 31.21 (2.968) | 13.41 (2.562) | 33.48 (3.103) |
| CI of mean | 25.33, 37.09 | 8.27, 18.55 | 27.33, 39.62 |
| Median (Range) | 20.28 (0.0, 98.6) | 9.03 (0.0, 97.3) | 16.33 (0.0, 98.6) |
CI=confidence interval, ITT=intent-to-treat, N=number of subjects, SE=standard error
In the analysis of the CDF (co-primary endpoint 1), a statistically significant difference between treatments (p <0.0001) was seen, which was in favour of Sixmo.
Figure 2. Cumulative Distribution Function of the Percentage of Urine Samples Negative for Opioids in Weeks 1-24 (co-primary endpoint 1), Study PRO-806 (ITT Population):
ITT=intent-to-treat, SL BPN = sublingual buprenorphine
Buprenorphine was not included in urine toxicology assessments.
The CDF results for co-primary endpoint 2 were fundamentally the same as for endpoint 1 (p <0.0001).
A key secondary endpoint in Study PRO-806 was the difference in proportions of urine samples that were negative for opioids over 24 weeks for Sixmo versus sublingual buprenorphine. Despite the use of an open-label comparator arm, this endpoint is considered robust, as it is based on urine toxicology. In this analysis, the percentage of opioid negative urines in the sublingual buprenorphine group was very similar to the results in the Sixmo group (33% versus 31%), and non-inferiority of Sixmo to sublingual buprenorphine was shown.
In Studies PRO-805 and PRO-806, 62.0% and 39.5% of Sixmo-treated subjects required supplemental SL buprenorphine. The mean doses per week in Sixmo subjects in PRO-805 and PRO-806 studies were 5.16 mg and 3.16 mg, with relatively low mean days of use per week of 0.45 and 0.31, respectively. In each of the two studies, the proportion of subjects requiring supplemental SL BPN was significantly higher in the placebo group than in the Sixmo group (90.9% and 66.7% of subjects, with mean days of use per week of 2.17 and 1.27, in PRO-805 and PRO-806, respectively). Retention in treatment was high in the Sixmo groups, with 65.7% and 64.0% of patients completing studies PRO-805 and PRO-806, respectively.
The majority of patients (around 80%) in both studies were adequately treated with 4 implants; around 20% of patients required a dose increase with a fifth implant.
In a subset of patients, Sixmo implants broke during implant removal. Breakage rates decreased in studies using the current technique and training. Generally, breakage was not perceived as a safety concern to the patient by the investigator.
Table 5. Implant breakage in Sixmo double-blind Phase 3 studies:
| Current technique and training | |||
|---|---|---|---|
| PRO-806 | PRO-811 | PRO-814 | |
| Sixmo N=99 | Sixmo N=78 | Sixmo N=82 | |
| Number (%) of broken implants | 71 (17.0%) | 81 (25.0%) | 35 (10.7%) |
| Number (%) of patients with broken implant(s) | 42 (42.4%) | 38 (48.7%) | 22 (26.8%) |
N=number of patients with data available.
Non-Caucasian population
The clinical experience with Sixmo in non-Caucasian patients is currently limited.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Sixmo in all subsets of the paediatric population for the maintenance treatment of opioid dependence (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
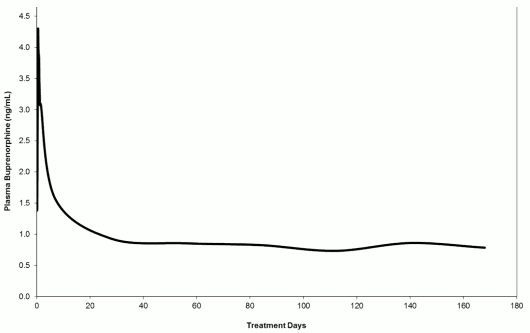
The Sixmo PK was assessed in opioid-dependent patients treated with Sixmo in studies TTP-400-02-01, PRO-810, PRO-805, PRO-806, PRO-807 and PRO-811. Prior to entry into acute studies PRO-805, PRO-806, PRO-810 and TTP-400-02-01, patients were treatment naïve adults, with moderate to severe opioid dependency. In the majority of patients, heroin was the primary opioid of use. After Sixmo implant insertion, an initial buprenorphine peak was observed and the median Tmax occurred at 12 hours after insertion. After the initial buprenorphine peak, the plasma buprenorphine concentrations decreased slowly and steady-state plasma buprenorphine concentrations were reached by approximately week 4. Mean steady-state plasma buprenorphine concentrations were consistent across all clinical studies, at approximately 0.5 to 1 ng/mL (with the 4-implant dose), and were maintained for approximately 20 weeks (week 4 through week 24) in a 24-week treatment period. At steady state, a small decrease in buprenorphine concentrations was also recorded between week 4 and week 24. Generally, concentrations were comparable to the trough buprenorphine concentration of 8 mg per day sublingual buprenorphine.
Plasma buprenorphine concentrations after Sixmo are illustrated in figure 3. Mean plasma buprenorphine concentrations up to day 28 are based on data from the relative bioavailability study PRO-810 (which had intensive PK sampling), while concentrations after day 28 are based on pooled data from studies PRO-805, PRO-806, PRO-807 and PRO-811.
Figure 3. Plasma buprenorphine concentrations after insertion of Sixmo (concentrations up to day 28 are based on study PRO-810, while concentrations after day 28 are based on studies PRO-805, PRO-806, PRO-807 and PRO-811):
Distribution
Buprenorphine is approximately 96% protein bound, primarily to alpha and beta globulin.
Biotransformation
Buprenorphine undergoes N-dealkylation to its major pharmacologically active metabolite norbuprenorphine and subsequent glucuronidation. The formation of norbuprenorphine was initially found to be performed by CYP3A4; subsequent studies also demonstrated the involvement of CYP2C8. Both buprenorphine and norbuprenorphine can further undergo glucuronidation by UDPglucuronosyltransferases.
Elimination
A mass balance study of buprenorphine showed complete recovery of radiolabel in urine (30%) and faeces (69%) collected up to 11 days after dosing. Almost all of the dose was accounted for in terms of buprenorphine, norbuprenorphine, and two unidentified buprenorphine metabolites. In urine, most of the buprenorphine and norbuprenorphine was conjugated (buprenorphine: 1% free and 9.4% conjugated; norbuprenorphine: 2.7% free and 11% conjugated). In faeces, almost all of the buprenorphine and norbuprenorphine were free (buprenorphine: 33% free and 5% conjugated; norbuprenorphine: 21% free and 2% conjugated).
Buprenorphine has a mean elimination half-life from plasma ranging from 24 to 48 hours.
Special populations
Hepatic impairment
The effect of hepatic impairment on the pharmacokinetics of Sixmo has not been studied. Buprenorphine is extensively metabolized in the liver and increased plasma levels were found to be increased in patients with moderate and severe hepatic impairment. Sixmo is contraindicated in patients with severe hepatic impairment.
Renal impairment
Renal elimination plays a relatively small role (approximately 30%) in the overall clearance of buprenorphine and buprenorphine plasma concentrations were not increased in patients with renal impairment. No Sixmo dose adjustment is therefore considered necessary for patients with renal impairment.
Elderly
Clinical studies of Sixmo did not include patients over 65 years; therefore, the use of the product in this population is not recommended. The efficacy and safety of buprenorphine in elderly patients >65 years has not been established.
5.3. Preclinical safety data
A standard battery of genotoxicity tests conducted on extracts of Sixmo and ethylene vinyl acetate (EVA) placebo implants was negative. Literature data indicated no genotoxic properties of buprenorphine.
There is no suspicion of carcinogenicity based on the clinical use of buprenorphine.
No published information is available regarding a potential effect of buprenorphine on male and female fertility. Studies in animals have shown reproductive toxicity.
When pregnant rats were exposed to buprenorphine through osmotic minipumps from gestation day 7 onwards, maternal food and water consumption was reduced on gestation days 7 to 20. The mortality index was significantly increased in the buprenorphine groups. There was a greater occurrence of resorptions and an increase in the number of stillbirths. Pups born tended to weigh less on postnatal day 1 compared with controls. Pups exposed to buprenorphine only during the prenatal period had a similar body weight compared with controls in the first 3 postnatal weeks. However, pups exposed to opioids postnatally exhibited significant body weights reductions. Maternal exposure to buprenorphine increased perinatal mortality and caused a delay in some development milestones in neonatal rats.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.