SOMAVERT Powder and solvent for solution for injection Ref.[9556] Active ingredients: Pegvisomant
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050, Bruxelles, Belgium
Therapeutic indications
Treatment of adult patients with acromegaly who have had an inadequate response to surgery and/or radiation therapy and in whom an appropriate medical treatment with somatostatin analogues did not normalize IGF-I concentrations or was not tolerated.
Posology and method of administration
Treatment should be initiated under the supervision of a physician experienced in the treatment of acromegaly.
Posology
A loading dose of 80 mg pegvisomant should be administered subcutaneously under medical supervision. Following this, SOMAVERT 10 mg reconstituted in 1 ml of solvent should be administered once daily as a subcutaneous injection.
Dose adjustments should be based on serum IGF-I levels. Serum IGF-I concentrations should be measured every four to six weeks and appropriate dose adjustments made in increments of 5 mg/day in order to maintain the serum IGF-I concentration within the age-adjusted normal range and to maintain an optimal therapeutic response.
Assessment of baseline levels of liver enzymes prior to initiation of SOMAVERT
Prior to the start of SOMAVERT, patients should have an assessment of baseline levels of liver tests (LTs) [serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum total bilirubin (TBIL), and alkaline phosphatase (ALP)]. For recommendations regarding initiation of SOMAVERT based on baseline LTs and recommendations for monitoring of LTs while on SOMAVERT, refer to Table A in Special warnings and precautionsfor use (4.4).
The maximum dose should not exceed 30 mg/day.
For the different dose regimens the following strengths are available: SOMAVERT 10 mg, SOMAVERT 15 mg, SOMAVERT 20 mg, SOMAVERT 25 mg and SOMAVERT 30 mg.
Paediatric population
The safety and efficacy of SOMAVERT in children aged 0 to 17 years have not been established. No data are available.
Elderly
No dose adjustment is required.
Hepatic or renal impairment
The safety and efficacy of SOMAVERT in patients with renal or hepatic insufficiency has not been established.
Method of administration
Pegvisomant should be administered by subcutaneous injection.
The site of injection should be rotated daily to help prevent lipohypertrophy.
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
Overdose
There is limited experience of overdose with pegvisomant. In the one reported incident of acute overdose, where 80 mg/day was administered for 7 days, the patient experienced a slight increase in fatigue and dry mouth. In the week following discontinuation of treatment the adverse reactions noted were: insomnia, increased fatigue, oedema peripheral, tremor, and weight gain. Two weeks after stopping treatment, leukocytosis and moderate bleeding from injection and vein puncture sites was observed which were considered possibly related to pegvisomant.
In cases of overdose, administration of this medicine should be discontinued and not resumed until IGF-I levels return to within or above the normal range.
Shelf life
Shelf life: 3 years.
After reconstitution, the product should be used immediately.
Special precautions for storage
Store the powder vial(s) in a refrigerator (2°C–8°C). Do not freeze. Keep the vial(s) in their outer carton in order to protect from light.
Store the pre-filled syringe(s) below 30C or store in a refrigerator (2°C-8°C). Do not freeze.
After reconstitution: For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
10 mg or 15 mg or 20 mg or 25 mg or 30 mg of pegvisomant in powder in a vial (type I flint glass) with a stopper (chlorobutylrubber) and 1 ml solvent (water for injections) in a pre-filled syringe (type I borosilicate glass) with a plunger stopper (bromobutyl rubber) and a tip cap (bromobutyl rubber). The colour of the protective plastic cap is specific to the strength of the product.
SOMAVERT 10 mg and 15 mg: Pack size of 30 vials, pre-filled syringes and safety needles.
SOMAVERT 20 mg, 25 mg and 30 mg: Pack sizes of 1 and 30 vial(s), pre-filled syringe(s) and safety needle(s)
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
The syringe and safety needle used to administer the injection are provided with the medicinal product.
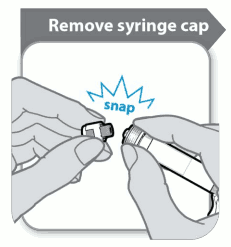
Before attaching the supplied safety needle the syringe cap will need to be removed from the pre-filled syringe. This is achieved by snapping it off. The syringe should be kept upright to avoid leakage and the end of the syringe should not be allowed to contact anything.
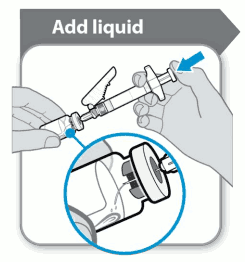
The powder should be reconstituted with 1 ml solvent. When adding the solvent from the syringe the vial and syringe should held at an angle as shown in the diagram below.
Add the solvent to the vial of powder. The solvent should be emptied into the vial slowly to avoid the possibility of a foam forming. This would make the medicine unusable. Gently dissolve the powder with a slow, swirling motion. Do not shake vigorously, as this might cause denaturation of the active substance.
After reconstitution, the reconstituted solution should be inspected visually for extraneous (or for any foreign) particulate matter or any variation in physical appearance prior to administration. In the event of either being observed, discard the medicinal product.
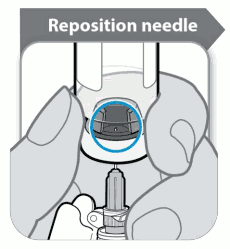
Before withdrawing the dissolved Somavert invert the vial with the syringe still inserted into it and ensure the gap in the stopper can be seen as shown in the diagram below:
Pull the needle down so that the needle tip is at its lowest point in the liquid. Slowly withdraw the plunger in the syringe to withdraw the medicine from the vial. If air is seen in the syringe, tap the barrel to float the bubbles to the top, and then gently push the bubbles out into the vial.
Before disposing of the syringe and needle fold the needle guard over the needle and ensure it clicks into place. The syringe and needle should never be reused.
For single use only. Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.