TEFIN Suppository Ref.[110140] Active ingredients: Butenafine Ibuprofen
Source: Health Products Regulatory Authority (IE) Revision Year: 2024 Publisher: Clonmel Healthcare Ltd, Waterford Road, Clonmel, Co. Tipperary, E91 D768, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: nonsteroidal anti-inflammatory and analgesic drugs
ATC Code: M01AE01
Ibuprofen is a nonsteroidal anti-inflammatory and analgesic active substance. In the commonly used animal models, its effect was shown to be due to the inhibition of prostaglandin synthesis. In humans, the active substance alleviates pain, swelling and fever caused by inflammation. Furthermore, ibuprofen inhibits the ADP and collagen-induced platelet aggregation.
Experimental data suggest that ibuprofen may competitively inhibit the effect of low dose acetylsalicylic acid on platelet aggregation when they are dosed concomitantly. Some pharmacodynamic studies show that when single doses of ibuprofen 400 mg were taken within 8 h before or within 30 min after immediate release acetylsalicylic acid dosing (81 mg), a decreased effect of acetylsalicylic acid on the formation of thromboxane or platelet aggregation occurred. Although there are uncertainties regarding extrapolation of these data to the clinical situation, the possibility that regular, long-term use of ibuprofen may reduce the cardioprotective effect of low-dose acetylsalicylic acid cannot be excluded. No clinically relevant effect is considered to be likely for occasional ibuprofen use (see section 4.5).
5.2. Pharmacokinetic properties
Orally given ibuprofen is partly absorbed in the stomach and completely absorbed from the small intestine. Following rectal administration, the active substance is almost completely absorbed, reaching plasma concentrations similar to those seen after oral ingestion. Peak plasma levels occur in one to two hours after oral administration. Ibuprofen is bound to plasma proteins to the extent of 99 per cent.
After metabolization in the liver (hydroxylation, carboxylation), the pharmacologically inactive metabolites are completely excreted, mainly through the kidneys (90 per cent) and partly in the bile. The elimination half-life is about two hours.
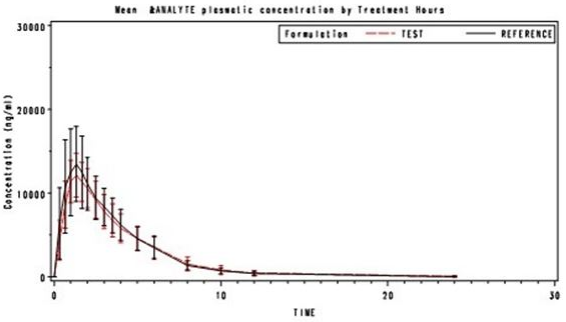
The following results were achieved in a bioavailability study performed in 2004 in 28 healthy volunteers, in comparison with a reference standard:
| Test preparation Tefin 150 milligram sup positories (ibuprofen) | Reference standard Ibuprofen suspension 150 milligram (oral) | |
|---|---|---|
| Peak plasma concentration (Cmax, µg/ml): | 12.8 ± 2.6 | 16.6 ± 3.1 |
| Time of peak plasma concentration (tmax, hr): Area under the plasma concentration-time curve (AUC, µg/ml*hr): | 1.443 ± 0.455 | 1.285 ± 0.635 |
| 53.94 ± 14.85 | 56.25 ± 12.68 |
These values are expressed (AUC0-inf) is 95.50% in relation to the oral suspension (reference standard)
Average course of plasma concentrations in comparison with a reference standard in a concentration-time diagram:
5.3. Preclinical safety data
The subchronic and chronic toxicity of ibuprofen in animal experiments showed up mainly in form of lesions and ulcerations in the gastro-intestinal tract. In vitro and in vivo studies gave no clinically relevant evidence of a mutagenic potential of ibuprofen. In studies in rats and mice no evidence of carcinogenic effects of ibuprofen was found.
Ibuprofen inhibited ovulation in rabbits and impaired implantation in different animal species (rabbit, rat, and mouse). Reproductive toxicity studies conducted in rats and rabbits have demonstrated that ibuprofen passes the placenta; for maternally toxic doses, an increased incidence of malformations (e.g. ventricular septal defects) was observed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.