TENSOPYN Tablet Ref.[51217] Active ingredients: Caffeine Codeine Doxylamine Paracetamol
Source: Health Products Regulatory Authority (ZA) Revision Year: 2023 Publisher: CIPLA MEDPRO (PTY) LTD., Building 9, Parc du Cap, Mispel Street, Bellville, 7530
4.1. Therapeutic indications
TENSOPYN tablets are indicated for mild to moderate pain associated with tension.
4.2. Posology and method of administration
Posology
Adults and children over 12 years: One to two tablets every 4 hours, with a maximum of 8 tablets daily.
DO NOT EXCEED THE RECOMMENDED DOSE.
Do not use continuously for longer than 5 days without consulting your doctor.
Special populations
Elderly population
Elderly patients may metabolise or eliminate opioid analgesics more slowly than younger adults, therefore dose reduction may be necessary (refer to section 4.4).
Renal & hepatic impairment
Patients suffering from liver or kidney disease should take paracetamol under medical supervision.
The dosage in renal functional impairment must be reduced.
Paediatric population
The safety and efficacy of TENSOPYN in children under 12 years has not yet been established.
Method of administration
TENSOPYN should be taken orally.
4.9. Overdose
Paracetamol
Prompt treatment is essential. In the event of an overdosage, consult a doctor immediately, or take the person to a hospital directly. A delay in starting treatment may mean that the antidote is given too late to be effective. Evidence of liver damage is often delayed until after the time for effective treatment has lapsed.
Susceptibility to paracetamol toxicity is increased in patients who have taken repeated high doses (greater than 5 -10 g/day) of paracetamol for several days, in chronic alcoholism, chronic liver disease, AIDS, malnutrition, and with the use of drugs that induce liver microsomal oxidation such as barbiturates, isoniazid, rifampicin, phenytoin and carbamazepine.
Symptoms of paracetamol overdosage in the first 24 hours include pallor, nausea, vomiting, anorexia and possibly abdominal pain.
Mild symptoms during the first two days of acute poisoning do not reflect the potential seriousness of the overdosage. Liver damage may become apparent 12 to 48 hours or later after ingestion, initially by elevation of the serum transaminase and lactic dehydrogenase activity, increased serum bilirubin concentration and prolongation of the prothrombin time. Liver damage may lead to encephalopathy, coma and death.
Acute renal failure with acute tubular necrosis may develop even in the absence of severe liver damage. Abnormalities of glucose metabolism and metabolic acidosis may occur. Cardiac arrhythmias have been reported.
Treatment for paracetamol overdosage
Although evidence is limited it is recommended that any adult person who has ingested 5-10 grams or more of paracetamol (or a child who has had more than 140 mg/kg) within the preceding four hours, should have the stomach emptied by lavage (emesis may be adequate for children). Ingestion of amounts of paracetamol smaller than this may require treatment in patients susceptible to paracetamol poisoning (see above).
N-acetylcysteine should be administered to all cases of suspected overdose as soon as possible preferably within eight hours of overdosage, although treatment up to 36 hours after ingestion may still be of benefit, especially if more than 150 mg/kg of paracetamol was taken. An initial dose of 150 mg/kg N-acetylcysteine in 200 mL dextrose injection given intravenously over 15 minutes, followed by an infusion of 50 mg/kg in 500 mL dextrose injection over the next four hours, and then 100 mg/kg in 1000 mL dextrose injection over the next sixteen hours. The volume of intravenous fluid should be modified for children.
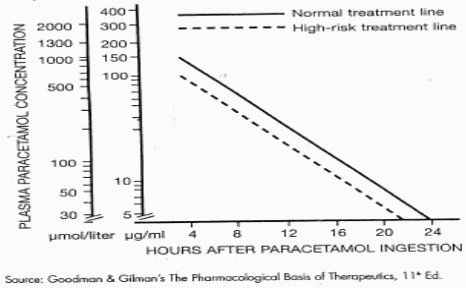
Although the oral formulation is not the treatment of choice, 140 mg/kg dissolved in water may be administered initially, followed by 70 mg/kg every four hours for seventeen doses. A plasma paracetamol level should be determined four hours after ingestion in all cases of suspected overdosage. Levels done before four hours, unless high, may be misleading. Patients at risk of liver damage, and hence requiring continued treatment with N- acetylcysteine, can be identified according to their plasma paracetamol level. The plasma paracetamol level can be plotted against time since ingestion in the normogram below. The nomogram should be used only in relation to a single acute ingestion.
Codeine phosphate
Overdosage produces central stimulation with exhilaration, in children, convulsions, followed by vomiting, drowsiness, respiratory depression and cyanosis, and coma.
Symptoms in decreasing order of frequency included somnolence, rash, miosis, vomiting, itching, ataxia, swelling of the skin and respiratory failure.
Opioid toxicity may occur in adults after overdoses of codeine tablets. Get emergency help immediately if any of the following symptoms occur:
Cold, clammy skin, confusion, convulsions, severe dizziness, low blood pressure, severe nervousness or restlessness, pinpoint pupils of eyes, slow heartbeat, slow or troubled breathing, severe weakness.
In the event of overdosage, consult a doctor or take the patient to the nearest hospital immediately.
In acute poisoning the stomach should be emptied by aspiration and lavage. Intensive supportive therapy may be necessary to correct respiratory failure and shock. The specific antagonist naloxone may be used to counteract severe respiratory depression.
Caffeine
Large doses may cause restlessness, excitement, muscle tremor, tinnitus, scintillating scotoma, tachycardia and extrasystoles.
Doxylamine succinate
Overdosage of doxylamine succinate causes sedation. Overdosage may be fatal, especially in infants and children in whom the main symptoms are central nervous system stimulation, and antimuscarinic effects, including ataxia, excitement, hallucinations, muscle tremors, convulsions, dilated pupils, dry mouth, flushed face and hyperpyrexia. Deepening coma, cardiorespiratory collapse and death may occur within 18 hours.
In adults the usual symptoms are central nervous system depression with drowsiness, coma and convulsions. Hypotension may also occur. Treatment of antihistamine overdose is symptomatic and supportive.
In the event of overdosage, consult a doctor or take the patient to the nearest hospital immediately. Respiratory depression will respond to naloxone administration.
Specialised treatment is essential as soon as possible. The latest information regarding the treatment of overdosage can be obtained from the nearest Poison Centre.
6.3. Shelf life
24 months.
6.4. Special precautions for storage
Store at or below 25°C.
Protect from moisture and light. Keep the container closed until ready for use.
Keep the tablets in the blister strips and outer carton until ready for use.
6.5. Nature and contents of container
TENSOPYN is packed in:
- Blister strips of 10 tablets packed in a carton containing 20 or 40 tablets.
- 40 tablets packed into white plastic containers with white plastic caps.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.