TIZVENI Concentrate for solution for infusion Ref.[110144] Active ingredients: Tislelizumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Beigene Ireland Limited, 10 Earlsfort Terrace, Dublin 2, D02 T380, Ireland, Tel. +353 1 566 7660, E-mail: bg.ireland@beigene.com
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Monoclonal antibodies and antibody drug conjugates
ATC code: L01FF09
Mechanism of action
Tislelizumab is a humanised immunoglobulin G4 (IgG4) variant monoclonal antibody against PD-1, binding to the extracellular domain of human PD-1. It competitively blocks the binding of both PD-L1 and PD-L2, inhibiting PD-1-mediated negative signalling and enhancing the functional activity in T cells in in vitro cell-based assays.
Clinical efficacy and safety
Non-small cell lung cancer
First-line treatment of non-squamous NSCLC: BGB-A317-304
BGB-A317-304 was a randomised, open-label, multicentre phase III study to investigate the efficacy and safety of tislelizumab in combination with platinum-pemetrexed versus platinum-pemetrexed alone as first-line treatment for chemotherapy-naïve patients with locally advanced non-squamous NSCLC who were not candidates for surgical resection or platinum-based chemoradiation, or metastatic non-squamous NSCLC.
The study excluded patients with active brain or leptomeningeal metastases, known EGFR mutations or ALK translocations sensitive to available targeted inhibitor therapy, active autoimmune disease, or any condition requiring systemic treatment with either corticosteroids (>10 mg daily of prednisone or equivalent) or other immunosuppressants.
A total of 334 patients were randomised (2:1) to receive tislelizumab 200 mg combined with pemetrexed 500 mg/m² and carboplatin AUC 5 mg/ml/min or cisplatin 75 mg/m² (T+PP arm, n=223) or pemetrexed 500 mg/m² and carboplatin AUC 5 mg/ml/min or cisplatin 75 mg/m² (PP arm, n=111). The choice of platinum (cisplatin or carboplatin) was at the investigator’s discretion.
The treatment was administered on a 3-week cycle. After the administration of 4, 5 or 6 cycles of chemotherapy or tislelizumab combined with chemotherapy at the investigator’s discretion, patients in the T+PP arm received tislelizumab 200 mg combined with pemetrexed 500 mg/m² on a 3-week cycle until disease progression or unacceptable toxicity; patients in the PP arm received pemetrexed 500 mg/m² alone until disease progression or unacceptable toxicity, and those with disease progression confirmed by Independent Review Committee (IRC) were given the option to cross over to receive tislelizumab monotherapy on a 3-week cycle.
Randomisation was stratified by PD-L1 expression in tumour cells (TC) (<1% versus 1% to 49% versus ≥50%) and disease stage (IIIB versus IV), as classified according to American Joint Committee on Cancer (AJCC), 7th edition of Cancer Staging Manual. PD-L1 expression was evaluated at a central laboratory using the Ventana PD-L1 (SP263) assay that identified PD-L1 staining on tumour cells. Tumour assessments were conducted every 6 weeks for the first 6 months, then every 9 weeks for the second 6 months, then every 12 weeks.
The baseline characteristics for patients in study BGB-A317-304 were: median age 61 years (range: 25 to 75), 29% age 65 years or older; 74% male; 100% Asian (all enrolled in China); 23.4% with ECOG PS of 0 and 76.6% with ECOG PS of 1; 18.3% with disease stage IIIB; 26.6% with unknown status for ALK rearrangement and 73.4% with negative ALK rearrangement; 36.2% never-smokers; 5.4% with brain metastases. The characteristics of age, sex, ECOG PS, stage, smoking status, PD-L1 TC score and prior anticancer treatments were balanced between the treatment arms.
The primary efficacy endpoint was progression-free survival (PFS) per RECIST v1.1 by IRC in the intent-to-treat (ITT) analysis. The secondary efficacy endpoints included overall survival (OS), objective response rate (ORR) and duration of response (DoR) per IRC and per investigator.
The study met its primary endpoint at the interim analysis (data cut-off date of 23-Jan-2020 and a median duration of study follow-up of 9.8 months), showing a statistically significant improvement in PFS with T+PP compared with PP. The stratified hazard ratio was 0.65 (95% CI: 0.47, 0.91; p=0.0054) with a median PFS of 9.7 months with T+PP and 7.6 months with PP.
The efficacy results of the final analysis (data cut-off date of 26-Oct-2020 and a median duration of study follow-up of 16.1 months) were consistent with those of the interim analysis.
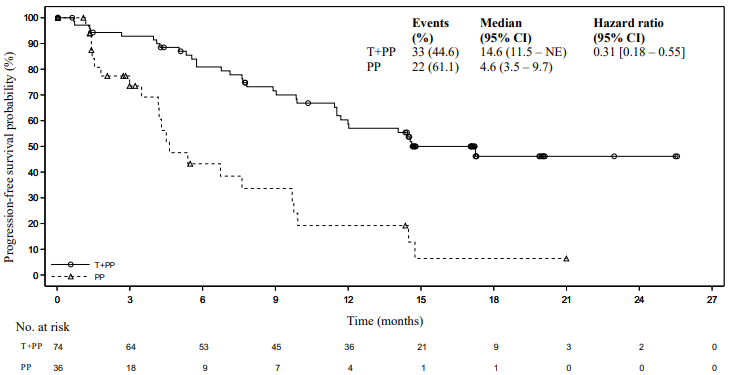
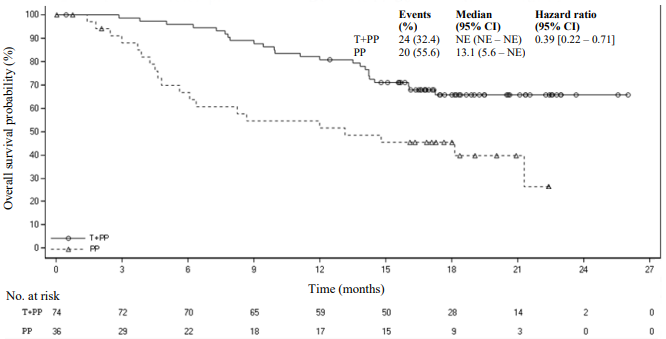
Amongst the 334 patients in study BGB-A317-304, 110 (33%) patients had tumour cell PD-L1 expression ≥50%. Of these, 74 patients were in the tislelizumab plus chemotherapy group and 36 patients were in the placebo plus chemotherapy group. Efficacy results of the patients with tumour cell PD-L1 expression ≥50% from the final analysis are shown in Table 3 and the Kaplan-Meier curve for PFS and OS is presented in Figures 1 and 2, respectively.
Table 3. Efficacy results in BGB-A317-304 in patients with PD-L1 expression ≥50%:
| Endpoint | Tislelizumab + Pemetrexed + Platinum (n=74) | Pemetrexed + Platinum (n=36) |
|---|---|---|
| PFS | ||
| Events, n (%) | 33 (44.6) | 22 (61.1) |
| Median PFS (months) (95% CI) | 14.6 (11.5, NE) | 4.6 (3.5, 9.7) |
| Stratified hazard ratioa (95% CI) | 0.31 (0.18, 0.55) | |
| OS | ||
| Deaths, n (%) | 24 (32.4) | 20 (55.6) |
| Median OS (months) (95% CI) | NE (NE, NE) | 13.1 (5.6, NE) |
| Stratified hazard ratioa (95% CI) | 0.39 (0.22, 0.71) | |
| Best overall response, n (%)b | ||
| ORRb, n (%) | 52 (70.3) | 11 (30.6) |
| 95% CIc | (58.5, 80.3) | (16.3, 48.1) |
| CR, n (%) | 7 (9.5) | 0 (0.0) |
| PR, n (%) | 45 (60.8) | 11 (30.6) |
| DoRb | ||
| Median DoR (months) (95% CI) | NE (13.2, NE) | 8.5 (3.3, NE) |
PFS = progression-free survival; CI = confidence interval; OS = overall survival; ORR = objective response rate; CR = complete response; PR = partial response; DoR = duration of response; NE = not estimable.
Medians were estimated by Kaplan-Meier method with 95% CIs estimated using the method of Brookmeyer and Crowley.
a Hazard ratio was estimated from stratified Cox model with pemetrexed+platinum group as reference group and stratified by disease stage (IIIB versus IV).
b PFS was based on IRC assessment, and ORR/DoR was based on the confirmed response by IRC.
c 95% CI was calculated using Clopper-Pearson method.
Figure 1. Kaplan Meier plot of PFS in BGB-A317-304 in patients with PD-L1 ≥50%:
Figure 2. Kaplan-Meier plot of OS in BGB-A317-304 in patients with PD-L1 ≥50%:
First-line treatment of squamous NSCLC: BGB-A317-307
BGB-A317-307 was a randomised, open-label, multicentre phase III study to compare the efficacy and safety of tislelizumab in combination with paclitaxel plus carboplatin or nab-paclitaxel plus carboplatin with that of paclitaxel plus carboplatin alone as first-line treatment for chemotherapy-naïve patients with locally advanced squamous NSCLC who were not candidates for surgical resection or platinum-based chemoradiation or metastatic squamous NSCLC.
The study excluded patients with active brain or leptomeningeal metastases, known EGFR mutations or ALK translocations sensitive to available targeted inhibitor therapy, active autoimmune disease, or any condition requiring systemic treatment with either corticosteroids (>10 mg daily of prednisone or equivalent) or other immunosuppressive treatments.
A total of 360 patients were randomised (1:1:1) to receive tislelizumab 200 mg combined with paclitaxel 175 mg/m² and carboplatin AUC 5 mg/ml/min (T+PC arm, n = 120), or tislelizumab 200 mg combined with nab-paclitaxel 100 mg/m² and carboplatin AUC 5 mg/ml/min (T+nPC arm, n=119), or paclitaxel 175 mg/m² and carboplatin AUC 5 mg/ml/min (PC arm, n=121).
The treatment was administered on a 3-week cycle, until the patient completed administration of 4 to 6 cycles of chemotherapy or tislelizumab combined with chemotherapy at the investigator’s discretion. Patients in the T+nPC and T+PC arms received tislelizumab until disease progression or unacceptable toxicity. Patients in the PC arm with disease progression were given the option to cross over to receive tislelizumab monotherapy on a 3-week cycle.
Randomisation was stratified by PD-L1 expression in tumour cells (TC) (<1% versus 1% to 49% versus ≥50%) and tumour staging (IIIB versus IV), as classified according to American Joint Committee on Cancer (AJCC), 7th edition of Cancer Staging Manual. PD-L1 expression was evaluated at a central laboratory using the Ventana PD-L1(SP263) assay that identified PD-L1 staining on tumour cells. Tumour assessments were conducted every 6 weeks for the first 6 months, then every 9 weeks for the remainder of the first year, then every 12 weeks until disease progression.
The baseline characteristics for the study population were: median age 62.0 years (range: 34 to 74), 35.3% age 65 years or older; 91.7% male; 100% Asian (all enrolled in China), 23.6% with ECOG PS of 0 and 76.4% with ECOG PS of 1; 33.9% diagnosed with stage IIIB and 66.1% with stage IV at baseline; 16.4% never-smokers; 38.3% with PD-L1 TC score <1%, 25.3% with PD-L1 TC score ≥1% and ≤49%, 34.7% with PD-L1 TC score ≥50%. The characteristics of age, sex, ECOG PS, stage, smoking status, PD-L1 TC score and prior anticancer treatments were balanced between the treatment arms.
The primary efficacy endpoint was progression-free survival (PFS) as assessed by IRC per RECIST v1.1 in the ITT analysis which was to be tested sequentially in arms T+PC versus PC and arms T+nPC versus PC. The secondary efficacy endpoints included overall survival (OS), objective response rate (ORR) and duration of response (DoR) per IRC and per investigator.
The study met its primary endpoint at the interim analysis (data cut-off date of 06-Dec-2019 and a median duration of study follow-up of 8.4 months), showing statistically significant improvements in PFS with tislelizumab in combination with paclitaxel and carboplatin (T+PC arm) and tislelizumab in combination with nab-paclitaxel and carboplatin (T+nPC arm) compared with paclitaxel and carboplatin alone (PC arm). The stratified HR (T+PC arm versus PC arm) was 0.48 (95% CI: 0.34, 0.69; p <0.0001). The stratified HR (T+nPC arm versus PC arm) was 0.45 (95% CI: 0.32, 0.64; p <0.0001). Median PFS was 7.6 months in the T+PC arm, 7.6 months in the T+nPC arm and 5.4 months in the PC arm.
The final analysis (data cut-off date of 30-Sep-2020 and a median duration of study follow-up of 16.7 months) showed the consistent results from the interim analysis.
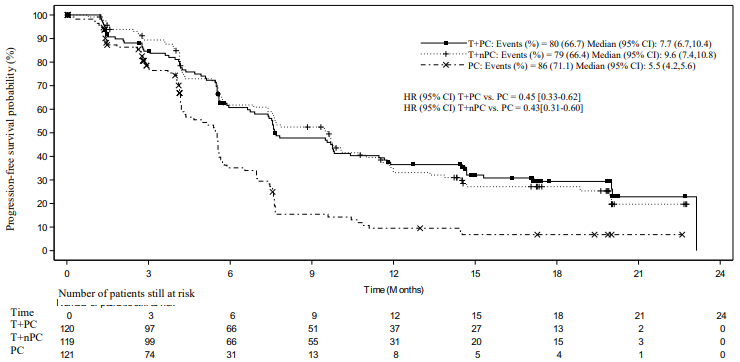
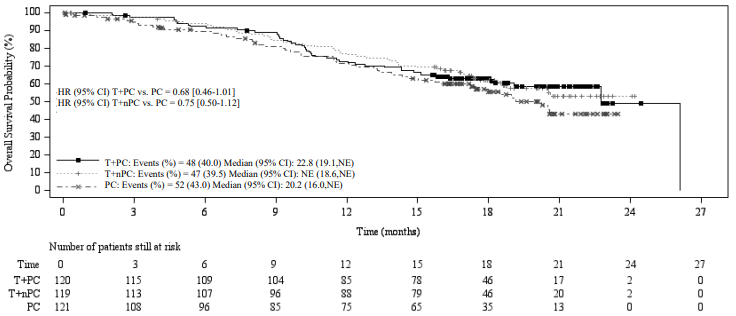
Efficacy results for the final analysis are shown in Table 4, Figure 3 and Figure 4.
Table 4. Efficacy results in BGB-A317-307:
| Endpoint | Tislelizumab + Paclitaxel + Carboplatin (n=120) | Tislelizumab + nab-Paclitaxel + Carboplatin (n=119) | Paclitaxel + Carboplatin (n=121) |
|---|---|---|---|
| PFS | |||
| Events, n (%) | 80 (66.7) | 79 (66.4) | 86 (71.1) |
| Median PFS (months) (95% CI) | 7.7 (6.7, 10.4) | 9.6 (7.4, 10.8) | 5.5 (4.2, 5.6) |
| Stratified hazard ratioa (95% CI) | 0.45 (0.33, 0.62) | 0.43 (0.31, 0.60) | - |
| OS | |||
| Deaths, n (%) | 48 (40.0) | 47 (39.5) | 52 (43.0) |
| Median OS (months) (95% CI) | 22.8 (19.1, NE) | NE (18.6, NE) | 20.2 (16.0, NE) |
| Stratified hazard ratio (95% CI) | 0.68 (0.45, 1.01) | 0.75 (0.50, 1.12) | - |
| ORRb | |||
| ORR, n (%) | 74 (61.7) | 74 (62.2) | 45 (37.2) |
| 95% CI | (52.4, 70.4) | (52.8, 70.9) | (28.6, 46.4) |
| CR, n (%) | 7 (5.8) | 6 (5.0) | 1 (0.8) |
| PR, n (%) | 67 (55.8) | 68 (57.1) | 44 (36.4) |
| DoRb | |||
| Median DoR (months) (95% CI) | 13.2 (7.85, 18.79) | 10.4 (8.34, 17.15) | 4.8 (4.04, 5.72) |
PFS = progression-free survival; CI = confidence interval; OS = overall survival; ORR = objective response rate; CR = complete response; PR = partial response; DoR = duration of response; NE = not estimable.
a Stratified by stratification factors: disease stage (IIIB versus IV) and PD-L1 expression in tumour cell (≥50% TC versus 1% to 49% TC versus <1% TC).
b PFS was based on IRC assessment, and ORR/DoR was based on the confirmed response by IRC.
Figure 3. Kaplan-Meier plot of PFS in BGB-A317-307 by IRC:
T+PC arm versus T+nPC arm versus PC arm
CI = Confidence interval; T+PC = tislelizumab+paclitaxel+carboplatin; T+nPC = tislelizumab+nab-paclitaxel+carboplatin; PC = paclitaxel+carboplatin.
Figure 4. Kaplan-Meier plot of OS in BGB-A317-307:
T+PC arm versus T+nPC arm versus PC arm
CI = Confidence interval; T+PC = tislelizumab+paclitaxel+carboplatin; T+nPC = tislelizumab+nab-paclitaxel+carboplatin; PC = paclitaxel+carboplatin; NE = not estimable.
Subgroup analyses demonstrated consistent PFS treatment effect across major demographic and prognostic subgroups, including PD-L1 expression <1%, 1 to 49% and ≥50% and disease stages IIIB and IV:
- for T+PC, with PFS HR of 0.57 (95% CI, HR = 0.34, 0.94) for PD-L1 <1%, 0.40 (95% CI, HR = 0.21, 0.76) for 1 to 49% and 0.44 (95% CI, HR = 0.26, 0.75)) for ≥50%
- for T+nPC, with PFS HR of 0.65 (95% CI, HR = 0.40, 1.06) for PD-L1 <1%, 0.40 (95% CI, HR = 0.22, 0.74) for 1 to 49% and 0.33 (95% CI, HR = 0.18, 0.59)) for ≥50%
Second-line treatment of NSCLC: BGB-A317-303
BGB-A317-303 was a randomised, open-label, multicentre phase III study to investigate the efficacy and safety of tislelizumab compared with docetaxel in patients with locally advanced or metastatic NSCLC (squamous or non-squamous), who had experienced disease progression on or after a prior platinum-based regimen.
The study excluded patients with known EGFR mutation or ALK rearrangement, prior PD-(L)1 inhibitor or CTLA-4 inhibitor treatment, active autoimmune disease, or any condition requiring systemic treatment with either corticosteroids (>10 mg daily of prednisone or equivalent) or other immunosuppressive treatments.
A total of 805 patients were randomised (2:1) ratio to receive tislelizumab 200 mg intravenously every 3 weeks (n=535) or docetaxel 75 mg/m² intravenously every 3 weeks (n=270). Randomisation was stratified by histology (squamous versus non-squamous), lines of therapy (second- versus third-line), and PD-L1 expression in tumour cells (TC) (≥25% versus <25%). Administration of docetaxel and tislelizumab continued until disease progression, as assessed by investigator per RECIST v1.1, or unacceptable toxicity. PD-L1 expression was evaluated at a central laboratory using the Ventana PDL1 (SP263) assay that identified PD-L1 staining on tumour cells. Tumour assessments were conducted every 9 weeks for 52 weeks after randomisation and continued every 12 weeks thereafter. Survival status was followed every 3 months after discontinuation of the study treatment.
The baseline characteristics for the study population were: median age 61 years (range: 28 to 88), 32.4% age 65 years or older, 3.2% age 75 years or older; 77.3% male; 17.0% White and 79.9% Asian; 20.6% with ECOG PS of 0 and 79.4% with ECOG PS of 1; 85.5% with metastatic disease; 30.3% never-smokers; 46.0% with squamous and 54.0% non-squamous histology; 65.8% with wild-type and 34% with unknown EGFR status; 46.1% with wild-type and 53.9% with unknown ALK status; 7.1% with previously treated brain metastases.
57.0% of the patients had a PD-L1 TC score <25% and 42.5% had a PD-L1 TC score ≥25%. All patients had received prior therapy with a platinum-doublet regimen: 84.7% patients received one prior therapy, 15.3% had received two prior therapies.
The dual-primary efficacy endpoints were OS in the ITT and PD-L1 TC score ≥25% analysis sets. Additional efficacy endpoints included investigator-assessed PFS, ORR and DoR.
BGB-A317-303 met both dual-primary endpoints of OS in the ITT analysis and PD-L1 ≥25% analysis sets. At the prespecified interim analysis (data cut-off date 10-Aug-2020 with a median duration of follow-up time of 11.7 months), a statistically significant improvement in OS was observed in the ITT population. Results favoured the tislelizumab arm (HR = 0.64; 95% CI: 0.53, 0.78; p<0.0001). Median OS was 17.2 months for the tislelizumab arm and 11.9 months for the docetaxel arm. At the final analysis (data cutoff date 15-Jul-2021 with a median duration of follow-up of 14.2 months), a statistically significant improvement in OS was observed in the PD-L1 ≥25% analysis set favouring the tislelizumab arm (stratified HR = 0.53; 95% CI: 0.41, 0.70; p<0.0001) with median OS being 19.3 months for the tislelizumab arm and 11.5 months for the docetaxel arm.
The final analysis (data cut-off date 15-Jul-2021 and a median duration of follow-up of 14.2 months) showed consistent efficacy results in the ITT population compared to the interim analysis.
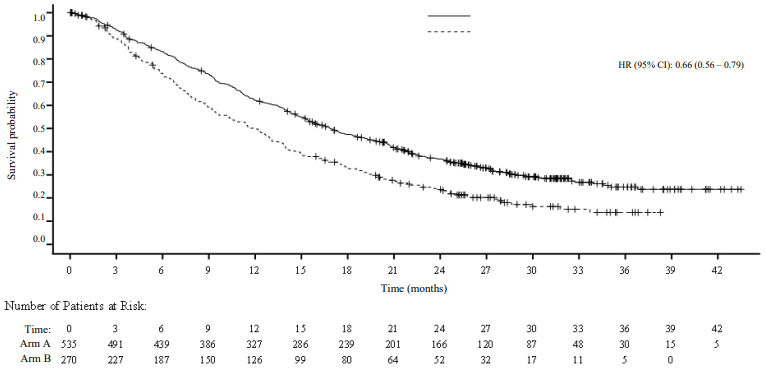
Table 5 and Figure 5 summarise the efficacy results for BGB-A317-303 (ITT analysis set) at the final analysis.
Table 5. Efficacy results in BGB-A317-303:
| Endpoint | Tislelizumab (n=535) | Docetaxel (n=270) |
|---|---|---|
| OS | ||
| Deaths, n (%) | 365 (68.2) | 206 (76.3) |
| Median OS (months) (95% CI) | 16.9 (15.24, 19.09) | 11.9 (9.63, 13.54) |
| Hazard ratio (95% CI),b | 0.66 (0.56, 0.79) | |
| PFS | ||
| Events, n (%) | 451 (84.3) | 208 (77.0) |
| Median PFS (months) (95% CI) | 4.2 (3.88, 5.52) | 2.6 (2.17, 3.78) |
| Hazard ratioa (95% CI) | 0.63 (0.53, 0.75) | |
| ORR (%) (95% CI)c | 20.9 (17.56, 24.63) | 3.7 (1.79, 6.71) |
| Best overall responsec | ||
| CR (%) | 1.7 | 0.4 |
| PR (%) | 19.3 | 3.3 |
| DoRc | ||
| Median DoR (months) (95% CI) | 14.7 (10.55, 21.78) | 6.2 (4.11, 8.31) |
OS = overall survival; CI = confidence interval; PFS = progression-free survival; ORR = objective response rate; CR = complete response; PR = partial response; DoR = duration of response.
Medians were estimated by Kaplan-Meier method with 95% CIs estimated using the method of Brookmeyer and Crowley.
a Hazard ratio was estimated from stratified Cox model with docetaxel group as reference group.
b Stratified by stratification factors: histology (squamous versus non-squamous), lines of therapy (second versus third), and PD-L1 expression in tumour cells (≥25% PD-L1 score versus <25% PD-L1 score).
c Confirmed by investigator.
Figure 5. Kaplan-Meier plot of OS in BGB-A317-303 (ITT Analysis Set):
Prespecified subgroup analyses demonstrated a consistent OS treatment effect in favour of tislelizumab across major demographic and prognostic subgroups.
Table 6 summarises efficacy results of OS by tumour PD-L1 (<25% TC, ≥25% TC) expression in prespecified subgroup analyses.
Table 6. Efficacy results of OS by tumour PD-L1 expression (<25% TC, ≥25% TC) in BGBA317-303:
| Tislelizumab arm | Docetaxel arm | |
|---|---|---|
| n=535 | n=270 | |
| PD-L1 expression in tumour cells <25%, n | 307 | 152 |
| Events, n (%) | 223 (72.6) | 117 (77.0) |
| Median OS (months) (95% CI) | 15.2 (13.4, 17.6) | 12.3 (9.3, 14.3) |
| Hazard ratioa (95% CI) | 0.79 (0.64, 0.99) | |
| PD-L1 expression in tumour cells ≥25%, n | 227 | 115 |
| Events, n (%) | 141 (62.1) | 86 (74.8) |
| Median OS (months) (95% CI) | 19.3 (16.5, 22.6) | 11.5 (8.2, 13.5) |
| Hazard ratioa (95% CI) | 0.54 (0.41, 0.71) | |
a Hazard ratio and its 95% CI were estimated from unstratified Cox model.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with tislelizumab in all subsets of the paediatric population in the treatment of malignant neoplasms (except central nervous system, haematopoietic and lymphoid tissue) (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
The pharmacokinetics (PK) of tislelizumab were assessed for Tizveni both as monotherapy and in combination with chemotherapy.
The PK of tislelizumab were characterised using population PK analysis with concentration data from 2 596 patients with advanced malignancies who received tislelizumab doses of 0.5 to 10 mg/kg every 2 weeks, 2.0 and 5.0 mg/kg every 3 weeks, and 200 mg every 3 weeks.
The time to reach 90% steady-state level is approximately 84 days (12 weeks) after 200 mg doses once every 3 weeks, and the steady-state accumulation ratio of tislelizumab PK exposure is approximately 2-fold.
Absorption
Tislelizumab is administered intravenously and therefore is immediately and completely bioavailable.
Distribution
A population pharmacokinetic analysis indicates that the steady-state volume of distribution is 6.42 l, which is typical of monoclonal antibodies with limited distribution.
Biotransformation
Tislelizumab is expected to be degraded into small peptides and amino acids via catabolic pathways.
Elimination
Based on population PK analysis, the clearance of tislelizumab was 0.153 l/day with an interindividual variability of 26.3% and the geometrical mean terminal half-life was approximately 23.8 days with a coefficient variation (CV) of 31%.
Linearity/non-linearity
At the dosing regimens of 0.5 mg/kg to 10 mg/kg once every 2 or 3 weeks (including 200 mg once every 3 weeks), the PK of tislelizumab were observed to be linear and the exposure was dose proportional.
Special populations
The effects of various covariates on tislelizumab PK were assessed in population PK analyses. The following factors had no clinically relevant effect on the exposure of tislelizumab: age (range 18 to 90 years), weight (range 32 to 130 kg), gender, race (White, Asian and other), mild to moderate renal impairment (creatinine clearance [CLCr] ≥30 ml/min), mild to moderate hepatic impairment (total bilirubin ≤3 times ULN and any AST), and tumour burden.
Renal impairment
No dedicated studies of tislelizumab have been conducted in patients with renal impairment. In the population PK analyses of tislelizumab, no clinically relevant differences in the clearance of tislelizumab were found between patients with mild renal impairment (CLCr 60 to 89 ml/min, n=1 046) or moderate renal impairment (CLCr 30 to 59 ml/min, n=320) and patients with normal renal function (CLCr ≥90 ml/min, n=1 223). Mild and moderate renal impairment had no effect on the exposure of tislelizumab (see section 4.2). Based on the limited number of patients with severe renal impairment (n=5), the effect of severe renal impairment on the pharmacokinetics of tislelizumab is not conclusive.
Hepatic impairment
No dedicated studies of tislelizumab have been conducted in patients with hepatic impairment. In the population PK analyses of tislelizumab, no clinically relevant differences in the clearance of tislelizumab were found between patients with mild hepatic impairment (bilirubin ≤ ULN and AST >ULN or bilirubin >1.0 to 1.5 x ULN and any AST, n=396) or moderate hepatic impairment (bilirubin >1.5 to 3 x ULN and any AST; n=12), compared to patients with normal hepatic function (bilirubin ≤ ULN and AST = ULN, n=2 182) (see section 4.2). Based on the limited number of patients with severe hepatic impairment (bilirubin >3 x ULN and any AST, n=2), the effect of severe hepatic impairment on the pharmacokinetics of tislelizumab is unknown.
5.3. Preclinical safety data
In repeat-dose toxicology studies in cynomolgus monkeys with intravenous dose administration at doses of 3, 10, 30 or 60 mg/kg every 2 weeks for 13 weeks (7 dose administrations), no apparent treatment-related toxicity or histopathological changes were observed at doses up to 30 mg/kg every 2 weeks, corresponding to 4.3 to 6.6 times the exposure in humans with the clinical dose of 200 mg.
No developmental and reproductive toxicity studies or animal fertility studies have been conducted with tislelizumab.
No studies have been performed to assess the potential of tislelizumab for carcinogenicity or genotoxicity.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.