WELIREG Film-coated tablet Ref.[108540] Active ingredients: Belzutifan
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
Belzutifan is an inhibitor of hypoxia-inducible factor 2 alpha (HIF-2α). HIF-2α is a transcription factor that plays a role in oxygen sensing by regulating genes that promote adaptation to hypoxia. Under normal oxygen levels, HIF-2α is targeted for ubiquitin-proteasomal degradation by VHL protein. Lack of functional VHL protein results in stabilization and accumulation of HIF-2α. Upon stabilization, HIF-2α translocates into the nucleus and interacts with hypoxia-inducible factor 1 beta (HIF-1β) to form a transcriptional complex that induces expression of downstream genes, including genes associated with cellular proliferation, angiogenesis, and tumor growth. Belzutifan binds to HIF-2α, and in conditions of hypoxia or impairment of VHL protein function, belzutifan blocks the HIF-2α-HIF-1β interaction, leading to reduced transcription and expression of HIF-2α target genes. In vivo, belzutifan demonstrated anti-tumor activity in mouse xenograft models of renal cell carcinoma.
12.2. Pharmacodynamics
Reductions in plasma levels of erythropoietin (EPO) were observed to be dose- and exposure-dependent at dosages up to 120 mg once daily. The maximum EPO suppression occurred following 2 weeks of consecutive dosing of WELIREG (mean percent decrease from baseline of approximately 60%). Mean EPO levels gradually returned to baseline values after 12 weeks of treatment.
The incidence of Grade 3 anemia increased with higher belzutifan exposure in patients with baseline hemoglobin levels <12 g/dL [see Warnings and Precautions (5.1)].
Cardiac Electrophysiology
At the recommended dosage, WELIREG does not cause large mean increases (i.e., >20 msec) in the QT interval.
12.3. Pharmacokinetics
The Cmax and AUC of belzutifan increase proportionally over a dose range of 20 mg to 120 mg WELIREG (0.17 to 1 times the approved recommended dose). The estimated geometric mean steady-state (CV%) Cmax is 1.5 μg/mL (45%) and AUC0-24h is 20.4 μg•hr/mL (62%) in patients treated with 120 mg WELIREG. Steady state is reached after approximately 3 days.
Absorption
The median Tmax occurs at 1 to 2 hours after WELIREG administration.
Effect of Food
A high-fat, high-calorie meal (total calories approximately 1000 kcal, 56 g fat, 55 g carbohydrate, and 31 g protein) delayed Tmax by approximately 2 hours with no clinically meaningful effect on Cmax, and AUC of belzutifan.
Distribution
The estimated mean (CV%) volume of distribution is 119 L (28%) Plasma protein binding of belzutifan is 45%. The blood-to-plasma concentration ratio of belzutifan is 0.88.
Elimination
The estimated mean (CV%) clearance is 6.0 L/hr (58%) and the mean elimination half-life is 14 hrs.
Metabolism
Belzutifan is primarily metabolized by UGT2B17 and CYP2C19 and to a lesser extent by CYP3A4 [see Clinical Pharmacology (12.5)].
Excretion
Following oral administration of radiolabeled belzutifan to healthy subjects, approximately 49.6% of the dose was excreted in urine and 51.7% in feces (primarily as inactive metabolites).
Specific Populations
Patients who are poor metabolizers of UGT2B17 and CYP2C19 had higher belzutifan AUC [see Clinical Pharmacology (12.5)].
There were no clinically significant differences in the pharmacokinetics of belzutifan based on age (19 to 90 years), sex, ethnicity (non-Hispanic, Hispanic), race (White, Black, Asian, Native American, Pacific Islander), body weight (42 to 166 kg), mild to moderate renal impairment (eGFR 30-89 mL/min/1.73 m² estimated by MDRD), or mild hepatic impairment (total bilirubin ≤ ULN with AST > ULN or total bilirubin > ULN to 1.5 x ULN with any AST). The effect of severe renal impairment (eGFR 15-29 mL/min/1.73 m²) and moderate to severe hepatic impairment (total bilirubin > 1.5 x ULN and any AST) have not been studied.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of Belzutifan on CYP3A Substrates: Coadministration of WELIREG 120 mg once daily with midazolam (a sensitive CYP3A4 substrate) decreased the midazolam AUC by 40% and the Cmax by 34%. Midazolam AUC is predicted to decrease up to 70% in patients with higher belzutifan concentrations (e.g., dual poor metabolizers) [see Clinical Pharmacology (12.5)].
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Belzutifan does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4.
Belzutifan does not induce CYP1A2 or CYP2B6.
Transporter Systems: Belzutifan is a substrate of P-gp, OATP1B1, and OATP1B3, but is not a substrate of BCRP.
Belzutifan inhibits MATE2K. Belzutifan does not inhibit P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT2, or MATE1.
12.5. Pharmacogenomics
Patients who are UGT2B17, CYP2C19, or dual UGT2B17 and CYP2C19 poor metabolizers are estimated to have 2.5-, 1.3-, or 3.2-fold higher belzutifan steady state AUC0-24h, respectively compared to patients who are UGT2B17 normal (extensive) metabolizers and CYP2C19 non-poor (ultrarapid, rapid, normal, and intermediate) metabolizers [see Use in Specific Populations (8.7)].
UGT2B17 poor metabolizers who are homozygous for the UGT2B17*2 allele have no UGT2B17 enzyme activity. CYP2C19 poor metabolizers (such as *2/*2, *3/*3, *2/*3) have significantly reduced or absent CYP2C19 enzyme activity. Approximately 15% of White, 6% of Black or African American, and up to 77% of certain Asian populations are UGT2B17 poor metabolizers. Approximately 2% of White, 5% of Black or African American, and up to 19% of certain Asian populations are CYP2C19 poor metabolizers. Approximately 0.4% of White, 0.3% of Black or African American, and up to 15% of certain Asian populations are dual UGT2B17 and CYP2C19 poor metabolizers.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with belzutifan.
Belzutifan was not mutagenic in the in vitro bacterial reverse mutation (Ames) assay. Belzutifan was not clastogenic in either an in vitro micronucleus assay or an in vivo rat bone marrow micronucleus assay.
Fertility studies in animals have not been conducted with belzutifan. In repeat-dose toxicity studies up to 3-month duration, belzutifan-related findings included degeneration/atrophy of testes and hypospermia and cellular debris of the epididymis in rats administered ≥2 mg/kg/day (approximately 0.1 times the human exposure at the recommended dose of 120 mg daily). Findings in testes and epididymis were associated with decreased sperm count and motility and abnormal sperm morphology at ≥6 mg/kg/day (approximately 0.2 times the human exposure at the recommended dose of 120 mg daily) and did not reverse by the end of the recovery period. Belzutifan had no adverse effects on female reproductive organs in repeat-dose toxicity studies up to 3-month duration; however, belzutifan caused embryo-fetal lethality (post-implantation loss) in pregnant rats given oral doses ≥60 mg/kg/day (approximately 1 time the human exposure at the recommended dose based on AUC) during the period of organogenesis [see Use in Specific Population (8.1)].
14. Clinical Studies
14.1 von Hippel-Lindau (VHL) disease
The efficacy of WELIREG was evaluated in LITESPARK-004 (NCT03401788), an open-label clinical trial in 61 patients with VHL-associated RCC diagnosed based on a VHL germline alteration and with at least one measurable solid tumor localized to the kidney as defined by response evaluation criteria in solid tumors (RECIST) v1.1. Enrolled patients had other VHL-associated tumors including CNS hemangioblastomas and pNET. CNS hemangioblastomas and pNET in these patients were diagnosed based on the presence of at least one measurable solid tumor in brain/spine or pancreas, respectively, as defined by RECIST v1.1 and identified by IRC. The study excluded patients with metastatic disease. Patients received WELIREG 120 mg once daily until progression of disease or unacceptable toxicity.
The study population characteristics were: median age 41 years [range 19-66 years], 3.3% age 65 or older; 53% male; 90% were White, 3.3% were Black or African-American, 1.6% were Asian, and 1.6% were Native Hawaiian or other Pacific Islander; 82% had an ECOG PS of 0, 16% had an ECOG PS of 1, and 1.6% had an ECOG PS of 2; and 84% had VHL Type I Disease. The median diameter of RCC target lesions per central independent review committee (IRC) was 2.2 cm (range 1-6.1). Median time from initial radiographic diagnosis of VHL-associated RCC tumors that led to enrollment on LITESPARK-004 to the time of treatment with WELIREG was 17.9 months (range 2.8-96.7). Seventy-seven percent of patients had prior surgical procedures for RCC.
The major efficacy endpoint for the treatment of VHL-associated RCC was objective response rate (ORR) measured by radiology assessment using Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 as assessed by IRC. Additional efficacy endpoints included duration of response (DoR), and time to response (TTR).
Table 6 summarizes the efficacy results for VHL-associated RCC in LITESPARK-004.
Table 6. Efficacy Results (IRC assessment) for LITESPARK-004 for VHL-Associated RCC:
| Efficacy Outcome Measure | WELIREG n=61 |
|---|---|
| Objective Response Rate, % (n) (95% CI) | 49% (30)* (36, 62) |
| Complete response | 0% |
| Partial response | 49% |
| Duration of Response | |
| Median in months (range) | Not reached (2.8+, 22+) |
| % (n) with DoR ≥12 months | 56% (17/30) |
+ Denotes ongoing response.
* All patients with a response were followed for a minimum of 18 months from the start of treatment.
For VHL-associated RCC, median TTR was 8 months (range 2.7, 19).
Table 7 summarizes the efficacy results for VHL-associated pNET or CNS hemangioblastomas in LITESPARK-004.
Table 7. Efficacy Results (IRC assessment) for LITESPARK-004 for VHL-Associated Subgroups with CNS Hemangioblastomas or pNET:
| Endpoint | Patients with CNS Hemangioblastomas n=24* | Patients with pNET n=12* |
|---|---|---|
| Objective Response Rate, % (n) (95% CI) | 63%, (15) (41, 81) | 83% (10) (52, 98) |
| Complete response | 4% (1) | 17% (2) |
| Partial response | 58% (14) | 67% (8) |
| Duration of Response | ||
| Median in months (range) | Not reached (3.7+, 22+) | Not reached (11+, 19+) |
| % (n) with DoR ≥12 months | 73% (11/15) | 50% (5/10) |
+ Denotes ongoing response.
* Number of patients with measurable solid lesions, based on IRC assessment.
For VHL-associated CNS hemangioblastomas, TTR was 3.1 months (range 2.5, 11). For VHL-associated pNET, median TTR was 8.1 months (range 2.7, 11).
Decreases in size of CNS hemangioblastoma-associated peri-tumoral cysts and syringes were observed.
14.2 Advanced Renal Cell Carcinoma (RCC)
The efficacy of WELIREG was evaluated in LITESPARK-005 (NCT04195750), an open-label, randomized, active-controlled clinical trial in 746 patients with unresectable, locally advanced or metastatic clear cell RCC that progressed following PD-1 or PD-L1 checkpoint inhibitor and VEGF receptor targeted therapies either in sequence or in combination.
Patients could have received up to 3 prior treatment regimens and were required to have measurable disease per RECIST v1.1. Patients were randomized in a 1:1 ratio to receive 120 mg WELIREG or 10 mg everolimus by oral administration once daily. Randomization was stratified by IMDC risk categories (favorable versus intermediate versus poor) and number of prior VEGF receptor targeted therapies (1 versus 2-3). Patients were evaluated radiologically at Week 9 from the date of randomization, then every 8 weeks through Week 49, and every 12 weeks thereafter.
The study population characteristics were: median age 63 years [range 22 to 90 years], 42% age 65 or older; 78% male; 79% White; 12% Asian; 1% Black or African American; 11% Hispanic or Latino; 44% ECOG performance status 0 and 55% ECOG performance status 1. Prior therapies: 13% patients had 1 prior line of therapy, 43% had 2 prior lines of therapy and 43% had 3 prior lines of therapy; 49% received 2 to 3 prior VEGF receptor targeted therapies. Patient distribution by IMDC risk categories was 22% favorable, 66% intermediate, and 12% poor. Common sites of metastasis in patients were 65% lung, 59% lymph nodes, and 49% bone.
The major efficacy endpoints were Progression Free Survival (PFS) measured by BICR using RECIST v1.1 and Overall Survival (OS). Additional efficacy endpoint included objective response rate (ORR) by BICR using RECIST v1.1.
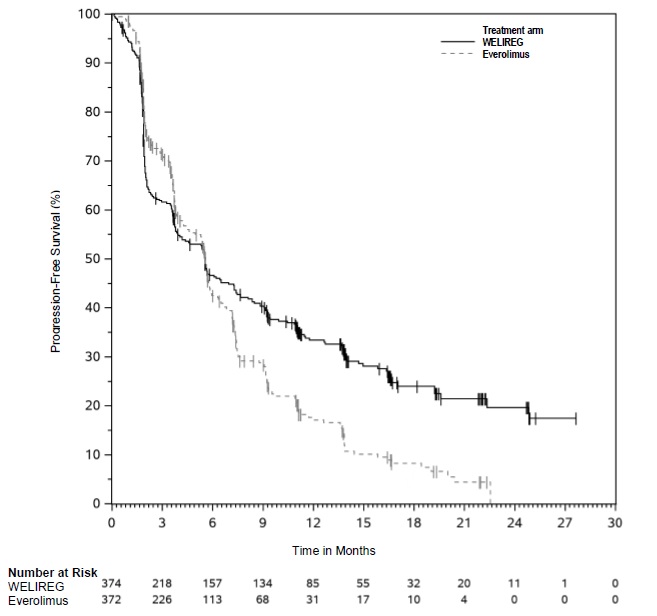
The trial demonstrated a statistically significant improvement in PFS for patients randomized to WELIREG compared with everolimus. Table 8 and Figure 1 summarize the efficacy results for LITESPARK-005.
Table 8. Efficacy Results for Advanced RCC (IRC assessment) for LITESPARK-005:
| Efficacy Outcome Measure | WELIREG n=374 | Everolimus n=372 |
|---|---|---|
| Progression-Free Survival (PFS) | ||
| Number of events, n (%) | 257 (69%) | 262 (70%) |
| Progressive disease | 234 (63%) | 222 (60%) |
| Death | 23 (6%) | 40 (11%) |
| Median in months (95% CI)* | 5.6 (3.9, 7.0) | 5.6 (4.8, 5.8) |
| Hazard ratio† (95% CI) | 0.75 (0.63, 0.90) | |
| p-Value‡ | 0.0008 | |
| Confirmed Objective Response Rate | ||
| Number of patients with measurable disease at baseline | 373 | 364 |
| ORR % (n) (95% CI) | 22% (82) (18, 27) | 4% (13) (2, 6) |
| Complete response | 3% (10) | 0% (0) |
| Partial response | 19% (72) | 4% (13) |
| p-Value§ | <0.0001 | |
* From product-limit (Kaplan-Meier) method for censored data
† Based on the stratified Cox proportional hazard model.
‡ One-sided p-Value based on stratified log-rank test compared with the significance boundary of 0.0021.
§ One-sided p-value based on stratified Miettinen and Nurminen (M&N) method.
Among the 82 patients treated with WELIREG who achieved a confirmed response based on BICR per RECIST 1.1, 25 (30%) patients had a duration of response ≥12 months. OS results were immature. At the time of the subsequent pre-specified analysis, 59% of the patients had died in the randomized population.
Figure 1. Kaplan-Meier Curve for Progression-Free Survival in LITESPARK-005:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.