XOLREMDI Capsule Ref.[109813] Active ingredients: Mavorixafor
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
Mavorixafor is an orally bioavailable CXC Chemokine Receptor 4 (CXCR4) antagonist [see Clinical Pharmacology (12.1)].
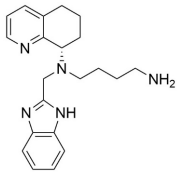
The chemical name of the active ingredient, mavorixafor, is N1-(1H-benzimidazol-2-ylmethyl)N1[(8S)-5,6,7,8-tetrahydroquinolin-8-yl]butane-1,4-diamine. It has a molecular formula of C21H27N5 and a molecular weight of 349.48 g/mol. Mavorixafor is of the S configuration and its structural formula is provided in Figure 1.
Figure 1. Structural Formula:
Mavorixafor is optically active and is a white to pale yellow to light brown solid. Mavorixafor is hygroscopic above relative humidities of 70%.
Mavorixafor is freely soluble in methanol, 95% ethanol and n-octanol, soluble in toluene, sparingly soluble in DMSO and acetonitrile, and very slightly soluble in HPLC grade water according to the USP solubility criteria.
Mavorixafor is soluble in pH 1.2 to 5.5 aqueous buffers and in pH 6.0 aqueous buffer, sparingly soluble in pH 6.8 aqueous buffer and slightly soluble in pH 7.5 aqueous buffer, according to the USP solubility criteria.
XOLREMDI is a hard gelatin capsule for oral administration. Each capsule contains 100 mg of mavorixafor with the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, microcrystalline cellulose, sodium lauryl sulfate, and sodium stearyl fumarate. The hard gelatin capsule contains FD&C Blue #2, gelatin, and titanium dioxide. The Black Ink contains ammonium hydroxide 28%, ferrosoferric oxide/black iron oxide (E172), isopropyl alcohol, n-butyl alcohol, propylene glycol, and shellac glaze in ethanol.
| Dosage Forms and Strengths |
|---|
|
Capsules: 100 mg, opaque hard gelatin capsules with white body and light blue cap. The white capsule body is imprinted with “100 mg” in black ink, and the light blue capsule cap is imprinted with “MX4” in black ink. |
| How Supplied |
|---|
|
XOLREMDI is supplied as an opaque white, hard gelatin capsule with a light blue cap, containing 100 mg of the active ingredient mavorixafor. The white capsule body is axially imprinted with “100 mg” in black ink, and the light blue capsule cap is axially imprinted with “MX4” in black ink. XOLREMDI is supplied in child-resistant bottles as follows:
Manufactured for: X4 Pharmaceuticals, Inc., Boston, MA 02134 |
Drugs
| Drug | Countries | |
|---|---|---|
| XOLREMDI | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.