XOLREMDI Capsule Ref.[109813] Active ingredients: Mavorixafor
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
Mavorixafor is an orally bioavailable CXC Chemokine Receptor 4 (CXCR4) antagonist that blocks the binding of the CXCR4 ligand, stromal-derived factor-1α (SDF-1α)/CXC Chemokine Ligand 12 (CXCL12). SDF-1/CXCR4 plays a role in trafficking and homing of leukocytes to and from the bone marrow compartment. Gain of function mutations in the CXCR4 receptor gene that occur in patients with WHIM syndrome lead to increased responsiveness to CXCL12 and retention of leukocytes in the bone marrow. Mavorixafor inhibits the response to CXCL12 in both wild-type and mutated CXCR4 variants associated with WHIM syndrome. Treatment with mavorixafor results in increased mobilization of neutrophils and lymphocytes from the bone marrow into peripheral circulation.
12.2. Pharmacodynamics
Absolute Neutrophil Count (ANC) and Absolute Lymphocyte Count (ALC)
ANC and ALC peaked at 4 hours after XOLREMDI dosing and returned towards baseline within 24 h after dosing. Over mavorixafor doses of 50 mg (0.125 times the maximum recommended dosage) to 400 mg once daily, higher mavorixafor exposure at steady state was associated with longer mean time (hours) above ANC threshold (TATANC) of 500 cells/μL and longer mean time (hours) above ALC threshold (TATALC) of 1,000 cells/μL over a 24-hour period.
Cardiac Electrophysiology
In a thorough QT (TQT) study, the maximum mean increase in the QTc interval was 15.6 ms (upper bound of the 90% confidence interval = 19.8 ms) after administration of XOLREMDI 800 mg (2 times the maximum recommended dose) in healthy volunteers [see Warnings and Precautions (5.2)]. In concentration-QT analysis the increase in the QTc interval was concentration-dependent.
12.3. Pharmacokinetics
Mavorixafor pharmacokinetic parameters are presented as geometric mean (CV%) in adults with WHIM syndrome unless otherwise specified. Mavorixafor steady state Cmax is 3304 (58.6%) ng/mL and the AUC from 0 to 24 hours (AUC0-24h) is 13970 (58.4%) ng*h/mL following 400 mg once daily.
Mavorixafor demonstrates nonlinear pharmacokinetics with greater than dose-proportional increases in Cmax and AUC0-24h over a dose range of 50 mg (0.125 times the recommended dosage) to 400 mg. Mavorixafor steady state is reached after approximately 9 to 12 days at the highest approved recommended dosage in healthy subjects.
Absorption
Mavorixafor median (range) time to Cmax (Tmax) is 2.8 hours (1.9 to 4 hours) at the highest approved recommended dosage.
Effect of Food
High Fat Meal: Mavorixafor Cmax decreased by 66% and AUC decreased by 55% following singledose administration of XOLREMDI 400 mg with a high-fat meal (1000 calories, 50% fat) to healthy subjects.
Low Fat Meal: Mavorixafor Cmax decreased by 55% and AUC decreased by 51% following singledose administration of XOLREMDI 400 mg with a low-fat meal (500 calories, 25% fat) to healthy subjects. In addition, a 14% higher mavorixafor Cmax and 18% lower AUC was observed following single-dose administration of XOLREMDI 400 mg with a low-fat meal to healthy subjects after an overnight fast compared to fasting for an additional 4 hours after the XOLREMDI dose.
Distribution
Mavorixafor volume of distribution is 768 L. Mavorixafor is >93% bound to human plasma proteins in vitro.
Elimination
Mavorixafor’s terminal half-life was 82 h (34%) with an apparent clearance of 62 L/h (40%) following single-dose administration of XOLREMDI 400 mg in healthy subjects. Mavorixafor exhibits at least partial nonlinear apparent clearance; however, this is not clinically significant at the approved recommended dosage.
Metabolism
CYP3A4 and, to a lesser extent, CYP2D6 are primarily responsible for mavorixafor metabolism.
Excretion
After a single oral dose of radiolabeled mavorixafor, 74.2% of the administered dose was recovered out of which 61.0% of administered radioactivity was recovered in feces and 13.2% (3% unchanged) was recovered in the urine over the 240-hour collection period in healthy subjects.
Specific Populations
No clinically significant differences in the pharmacokinetics of mavorixafor were observed based on age (12 to 58 years), sex, or mild to moderate renal impairment (CLcr 30 to <90 mL/min as estimated by the Cockcroft-Gault formula). The effects of severe renal impairment (CLcr 15 to <30 mL/min), end-stage renal disease (CLcr <15 mL/min), and moderate to severe hepatic impairment on the pharmacokinetics of mavorixafor are unknown.
Lower body weight was associated with lower mavorixafor clearance. Mavorixafor exposures in patients with WHIM syndrome are comparable between those weighing 50 kg or less who receive 300 mg once daily and those weighing greater than 50 kg who receive 400 mg once daily. Following 400 mg once daily, median Cmax and AUC is 22% and 30% lower, respectively, in patients with higher body weight (85 kg and above) compared to patients with average body weight (50 to less than 85 kg). The difference in exposure between patients with average body weight and patients with higher body weight is not expected to have a clinically significant impact on patient outcomes.
Pediatric Patients
No clinically significant differences in the pharmacokinetics of mavorixafor were identified in pediatric patients aged 12 to <17 years with WHIM syndrome compared to adult patients after accounting for differences in body weight.
Drug Interaction Studies
Clinical Studies
Strong CYP3A4 Inhibitors: The systemic exposure of mavorixafor following concomitant administration of a single dose of XOLREMDI 200 mg (0.5 times the recommended dosage for adult and adolescent patients 12 years and older weighing over 50 kg) with 200 mg itraconazole (strong CYP3A4 inhibitor and P-gp inhibitor) dosed to steady state was similar to the mavorixafor systemic exposure from a single dose of XOLREMDI 400 mg administered alone in healthy subjects. These results suggest an approximate increase in mavorixafor exposure by 2-fold due to itraconazole.
CYP2D6 Substrates: Dextromethorphan (CYP2D6 substrate) Cmax increased by 6-fold (CI90%: 5.1 to 8.3) and AUC increased by 9-fold (CI90%: 6.5 to 12.3) following concomitant use with XOLREMDI 400 mg in healthy subjects.
CYP3A4 Substrates: Midazolam (CYP3A4 substrate) Cmax increased by 1.1-fold (CI90%: 1.0 to 1.3) and AUC increased by 1.7-fold (CI90%: 1.4 to 2.1) following concomitant use with XOLREMDI 400 mg in healthy subjects.
P-gp Substrates:
Digoxin: Digoxin Cmax increased by 1.5-fold (CI90%: 1.3 to 1.8) and AUC increased by 1.6-fold (CI90%: 1.4 to 1.9) following concomitant use of a single oral dose of a transporter cocktail containing 0.25 mg of digoxin with XOLREMDI dosed to steady state (400 mg/day) in healthy subjects.
Metformin: Metformin Cmax decreased by 35% (CI90%: 17 to 49%) and AUC decreased by 35% (CI90%: 20 to 47%) following concomitant use of a single oral dose of a transporter cocktail containing 10 mg of metformin with XOLREMDI dosed to steady state (400 mg/day) in healthy subjects.
Other Drugs: No clinically significant differences in the pharmacokinetics of caffeine (CYP1A2 substrate), losartan (CYP2C9 substrate), omeprazole (CYP2C19 substrate), furosemide (OAT1 and OAT3 substrate) and oral contraceptives were observed following concomitant use with mavorixafor.
In Vitro Studies
CYP450 Enzymes: Mavorixafor is a substrate of CYP3A4, CYP2D6, CYP3A5, and CYP2C19, but is not a substrate of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C19, CYP2E1, and CYP4A11. Mavorixafor is an inhibitor of CYP3A4 (time dependent), CYP3A5, CYP2D6, CYP2B6, CYP1A2, CYP2C8, CYP2C9, and CYP2C19. Mavorixafor is an inducer of CYP1A2, but not an inducer of CYP2B6, CYP2C8, CYP2C9 and CYP3A4.
Transporter Systems: Mavorixafor is a substrate of P-gp, but not a substrate of OATP1B1, OATP1B3, OAT1, OAT3, OCT2, MATE1 or MATE2-K. Mavorixafor is an inhibitor of P-gp, OCT2, and MATE1, but is not an inhibitor of BCRP, OATP1B1, OATP1B3, OAT1, OAT3, and MATE2-K.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with mavorixafor have not been conducted.
Mavorixafor was not genotoxic in an in vitro bacterial reverse mutation assay (Ames test), in an in vitro human lymphocyte culture chromosome aberration assay, or in an in vivo rat bone marrow micronucleus assay.
Fertility studies have not been conducted with mavorixafor. Significant tubular degeneration/atrophy was observed in the testes of dogs at clinical exposure.
14. Clinical Studies
The efficacy of XOLREMDI in patients aged 12 years and older with WHIM syndrome was demonstrated in the 52-week, randomized, double-blind, placebo-controlled portion of Study 1 [NCT03995108]. Enrolled patients had a genotype-confirmed variant of CXCR4 consistent with WHIM syndrome, and a confirmed absolute neutrophil count (ANC) ≤400 cells/µL. Patients were permitted to continue (but not initiate) immunoglobulin therapy at the same dose. Use of other CXCR4 antagonists was not permitted. Baseline patient demographics are shown in Table 2.
Table 2. Baseline Demographics and Disease Characteristics in Patients with WHIM Syndrome (Study 1):
| Demographics and Disease Characteristics | XOLREMDI (N=14) | Placebo (N=17) |
|---|---|---|
| Demographics | ||
| Age (years) Mean (SD) | 22.1 (12.20) | 30.9 (21.25) |
| Age group, n (%) | ||
| 12 to <18 years | 7 (50) | 8 (47.1) |
| ≥18 years | 7 (50) | 9 (52.9) |
| Sex , n (%) | ||
| Male | 5 (35.7) | 8 (47.1) |
| Female | 9 (64.3) | 9 (52.9) |
| Race, n (%) | ||
| White | 13 (93) | 16 (94) |
| Asian | 0 | 1 (6) |
| Other | 1 (7) | 0 |
| Ethnicity, n (%) | ||
| Not Hispanic or Latino | 13 (93) | 17 (100) |
| Hispanic or Latino | 1 (7) | 0 |
| Disease Characteristics | ||
| Baseline Ig use, n (%) | ||
| Yes | 6 (42.9) | 8 (47.1) |
| Baseline mean absolute neutrophil count (ANC) (cells/µL) Mean (SD) | 155 (93.8) | 281 (232.7) |
| Baseline mean absolute lymphocyte count (ALC) (cells/µL) Mean (SD) | 501 (204.8) | 563 (199.1) |
Abbreviations: SD = standard deviation; Ig = immunoglobulin.
Note: Percentages are calculated based on the number of patients within each characteristic as denominator.
Thirty-one patients were randomized 1:1 to receive either placebo (N=17) or XOLREMDI (N=14) once daily for 52 weeks. The efficacy of XOLREMDI in the treatment of patients with WHIM syndrome was based on improvement in absolute neutrophil counts (ANC), improvement in absolute lymphocyte counts (ALC), and a reduction in infections.
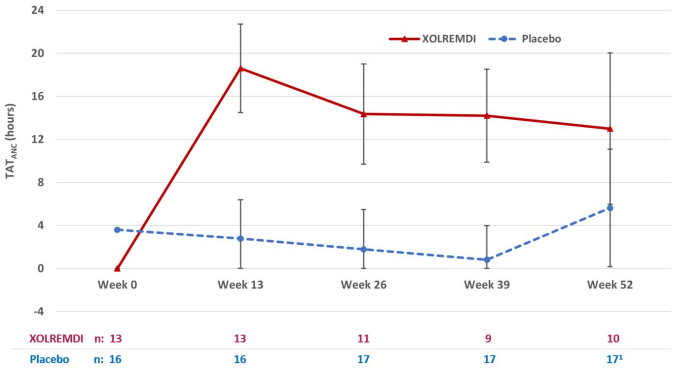
For ANC, the mean time (hours) above ANC threshold (TATANC) of 500 cells/μL over a 24-hour period was assessed 4 times throughout the study (every 3 months for 12 months). The results over the 52-week period showed that TATANC was statistically significantly greater in patients treated with XOLREMDI (LS mean [SE] 15.0 [1.89] hours) compared with placebo (2.8 [1.52] hours) (p value <0.0001) (see Table 3 and Figure 2).
Table 3. Mean Time (hours) Above ANC Threshold (TATANC) in Study 1:
| XOLREMDI (N=14) | Placebo (N=17) | ||
|---|---|---|---|
| TATANC (hours) | |||
| Baseline | Mean (SD) | 0.0 (0.0) | 3.6 (5.7) |
| Overall MMRM results | LS mean (SE) | 15.0 (1.89) | 2.8 (1.52) |
| LS mean 95% CI | (11.2, 18.9) | (0.0, 5.9) | |
| Difference from placebo: | |||
| LS mean difference (SE) | 12.3 (2.49) | - | |
| LS mean difference 95% CI | (7.2, 17.4) | - | |
| P-value1 | <0.0001 | - | |
Abbreviations: ANC = absolute neutrophil count; CI = confidence interval; LS = least squares; MMRM = mixed-model repeated measures; SD = standard deviation; SE = standard error; TAT = time above threshold of 500 cells/μL.
1 The results are based on an MMRM analysis with time above threshold as a dependent variable; treatment, visit (Weeks 13, 26, 39 and 52), treatment*visit, Ig use (randomization strata), and baseline time above threshold as covariates; and patient as the repeated random effect.
Figure 2. TATANC Over Time (Hours) (LS Mean ± 95% CI) by Treatment Group (Study 1):
Abbreviations: ANC = absolute neutrophil count; CI = confidence interval; LS = least squares; TAT = total time (hours) above threshold (500 cells/µL) in 24 hours.
1 At Week 52, 3 of 17 placebo patients were given XOLREMDI in advance of their TAT measurements as they entered the open-label period of the study; one XOLREMDI patient did not take XOLREMDI. All data were included in the ITT analysis.
For ALC, the mean time (hours) above ALC threshold (TATALC) of 1,000 cells/μL over a 24-hour period was assessed 4 times throughout the study (every 3 months for 12 months). The results over the 52-week period showed that TATALC was statistically significantly greater in patients treated with XOLREMDI (LS mean [SE] 15.8 [1.39] hours) compared with placebo (4.6 [1.15] hours) (p value <0.0001).
The efficacy of XOLREMDI was further assessed in a composite endpoint consisting of total infection score and total wart change score using a Win-Ratio method (Table 4). The Win-Ratio of 2.76 is the number of pairs of XOLREMDI-treated patient “wins” divided by the number of pairs of placebo patient “wins”.
Table 4. Win-Ratio Analysis* for the Composite Clinical Efficacy Endpoint Based on Total Infection Score and Total Wart Change Score:
| Category | n** | Win-Ratio (95% CI) |
|---|---|---|
| XOLREMDI wins on total infection score | 174 | 2.76 (1.60, 4.76) |
| Placebo wins on total infection score | 63 | |
| XOLREMDI wins on total wart change score | 0 | |
| Placebo wins on total wart change score | 0 | |
| None of the above (tie) | 1 |
* The method compared each XOLREMDI-treated patient to each placebo-treated patient in a pair-wise manner that proceeded in a hierarchical fashion using total infection score, followed by total wart change score if patients could not be differentiated based on total infection score. The total infection score was calculated by summing up the number of infection events weighted by severity and divided by the total exposure time (in years). Total wart change score was calculated by summing up the regional wart change scores from 3 target regions (lesions).
** n is number of wins.
Analyses of the individual components of this composite endpoint showed an approximately 40% reduction of total infection score, weighted by infection severity, in XOLREMDI-treated patients compared with placebo-treated patients. The annualized infection rate was reduced approximately 60% in XOLREMDI-treated patients [LS mean (SE) 1.7(0.5)] compared with placebo-treated patients [LS mean (SE) 4.2 (0.7)]. There was no difference in total wart change scores between the XOLREMDI and placebo treatment arms over the 52-week period.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.