ZEPBOUND Solution for injection Ref.[109319] Active ingredients: Tirzepatide
Source: FDA, National Drug Code (US) Revision Year: 2023
1. Indications and Usage
ZEPBOUND is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index (BMI) of:
- 30 kg/m2 or greater (obesity) or
- 27 kg/m2 or greater (overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, dyslipidemia, type 2 diabetes mellitus, obstructive sleep apnea, or cardiovascular disease).
Limitations of Use
- ZEPBOUND contains tirzepatide. Coadministration with other tirzepatide-containing products or with any glucagon-like peptide-1 (GLP-1) receptor agonist is not recommended.
- The safety and efficacy of ZEPBOUND in combination with other products intended for weight management, including prescription drugs, over-the-counter drugs, and herbal preparations, have not been established.
- ZEPBOUND has not been studied in patients with a history of pancreatitis [see Warnings and Precautions (5.5)].
2. Dosage and Administration
2.1 Patient Selection
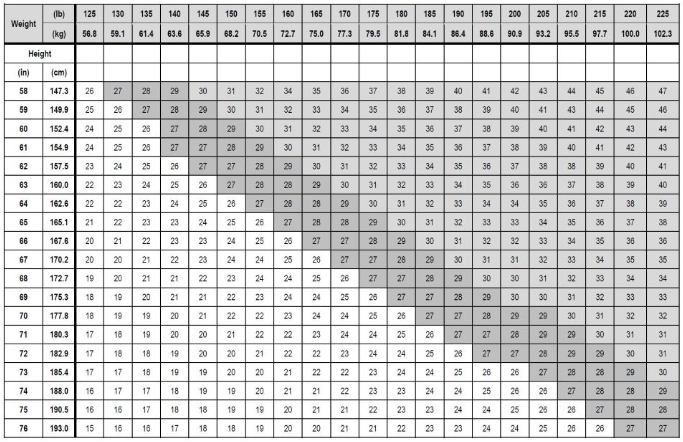
Select adult patients for ZEPBOUND treatment as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management based on their BMI. Table 1 presents a chart for determining BMI based on height and weight. BMI is calculated by dividing weight (in kilograms) by height (in meters) squared.
Table 1. BMI Conversion Chart:
2.2 Recommended Dosage
- The recommended starting dosage of ZEPBOUND is 2.5 mg injected subcutaneously once weekly. The 2.5 mg dosage is for treatment initiation and is not intended for chronic weight management.
- After 4 weeks, increase the dosage to 5 mg injected subcutaneously once weekly.
- The dosage may be increased in 2.5 mg increments, after at least 4 weeks on the current dose.
- The recommended maintenance dosages of ZEPBOUND in adults are 5 mg, 10 mg, or 15 mg injected subcutaneously once weekly.
- Consider treatment response and tolerability when selecting the maintenance dosage. If patients do not tolerate a maintenance dosage, consider a lower maintenance dosage.
- The maximum dosage of ZEPBOUND is 15 mg injected subcutaneously once weekly.
2.3 Recommendations Regarding Missed Dose
- If a dose is missed, instruct patients to administer ZEPBOUND as soon as possible within 4 days (96 hours) after the missed dose. If more than 4 days have passed, skip the missed dose and administer the next dose on the regularly scheduled day. In each case, patients can then resume their regular once weekly dosing schedule.
- The day of weekly administration can be changed, if necessary, as long as the time between the two doses is at least 3 days (72 hours).
2.4 Important Administration Instructions
- Prior to initiation of ZEPBOUND, train patients and caregivers on proper injection technique. Refer to the accompanying Instructions for Use for complete administration instructions with illustrations.
- Inspect ZEPBOUND visually before use. It should appear clear and colorless to slightly yellow. Do not use ZEPBOUND if particulate matter or discoloration is seen.
- Administer ZEPBOUND once weekly at any time of day, with or without meals.
- Inject ZEPBOUND subcutaneously in the abdomen, thigh, or upper arm.
- Rotate injection sites with each dose.
10. Overdosage
In the event of an overdosage, contact the Poison Help Line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations. Appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms. A period of observation and treatment for these symptoms may be necessary, taking into account the half-life of tirzepatide of approximately 5 days.
16.2. Storage and Handling
- Store ZEPBOUND in a refrigerator at 2°C to 8°C (36°F to 46°F).
- If needed, each single-dose pen can be stored unrefrigerated at temperatures not to exceed 30°C (86°F) for up to 21 days. If ZEPBOUND is stored at room temperature, it should not be returned to the refrigerator.
- Discard if not used within 21 days after removing from the refrigerator.
- Do not freeze ZEPBOUND. Do not use ZEPBOUND if frozen.
- Store ZEPBOUND in the original carton to protect from light.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.