LUTATHERA Solution for injection Ref.[109757] Active ingredients: Lutetium ¹⁷⁷Lu oxodotreotide
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
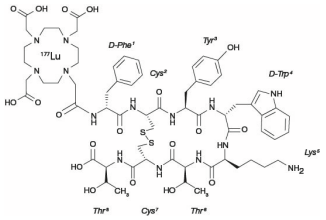
Lutetium Lu 177 dotatate is a radiolabeled somatostatin analog. The drug substance lutetium Lu 177 dotatate is a cyclic peptide linked with the covalently bound chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid to a radionuclide.
Lutetium Lu 177 dotatate is described as lutetium (Lu 177)N[(4,7,10-Tricarboxymethyl-1,4,7,10-tetraazacyclododec-1-yl) acetyl]-D-phenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophanyl-L-lysyl-L-threoninyl-L-cysteinyl-L-threonine-cyclic (2-7) disulfide. The molecular weight is 1609.6 Daltons and the structural formula is as follows:
LUTATHERA (lutetium Lu 177 dotatate) 370 MBq/mL (10 mCi/mL) Injection is a sterile, clear, colorless to slightly yellow solution for intravenous use. Each single-dose vial contains acetic acid (0.48 mg/mL), sodium acetate (0.66 mg/mL), gentisic acid (0.63 mg/mL), sodium hydroxide (0.64 mg/mL), ascorbic acid (2.8 mg/mL), diethylene triamine pentaacetic acid (0.05 mg/mL), sodium chloride (6.85 mg/mL), and Water for Injection (ad 1 mL). The pH range of the solution is 4.5 to 6.
11.1 Physical Characteristics
Lutetium-177 decays to stable hafnium-177 with a half-life of 6.647 days, by emitting beta-minus radiation with a maximum energy of 0.498 MeV (79%) and photonic radiation (γ) of 0.208 MeV (11%) and 0.113 MeV (6.2%). The main radiations of lutetium-177 are detailed in Table 7.
Table 7. Lutetium-177 Main Radiations:
| Radiation | Energy (keV) | Iβ-% | Iγ% |
|---|---|---|---|
| β- | 176.5 | 12.2 | |
| β- | 248.1 | 0.05 | |
| β- | 384.9 | 9.1 | |
| β- | 497.8 | 78.6 | |
| γ | 71.6 | 0.15 | |
| γ | 112.9 | 6.20 | |
| γ | 136.7 | 0.05 | |
| γ | 208.4 | 11.0 | |
| γ | 249.7 | 0.21 | |
| γ | 321.3 | 0.22 |
11.2 External Radiation
Table 8 summarizes the radioactive decay properties of lutetium-177.
Table 8. Physical Decay Chart: Lutetium-177 Physical Half-Life = 6.647 Days:
| Hours | Fraction remaining | Hours | Fraction remaining |
|---|---|---|---|
| 0 | 1.000 | 48 (2 days) | 0.812 |
| 1 | 0.996 | 72 (3 days) | 0.731 |
| 2 | 0.991 | 168 (7 days) | 0.482 |
| 5 | 0.979 | 336 (14 days) | 0.232 |
| 10 | 0.958 | 720 (30 days) | 0.044 |
| 24 (1 day) | 0.901 | 1080 (45 days) | 0.009 |
| Dosage Forms and Strengths |
|---|
|
Injection: 370 MBq/mL (10 mCi/mL) of lutetium Lu 177 dotatate as a clear and colorless to slightly yellow solution in a single-dose vial. |
| How Supplied |
|---|
|
LUTATHERA Injection containing 370 MBq/mL (10 mCi/mL) of lutetium Lu 177 dotatate is a sterile, preservative-free and clear, colorless to slightly yellow solution for intravenous use supplied in a clear, colorless Type I glass 30 mL single-dose vial containing 7.4 GBq (200 mCi) ± 10% of lutetium Lu 177 dotatate at the time of injection (NDC# 69488-003-01). The solution volume in the vial ranges between 20.5 to 25 mL to provide a total of 7.4 GBq (200 mCi) of radioactivity. The product vial is enclosed within a lead shielded container (NDC# 69488-003-01) placed in a plastic sealed container. The product is shipped in a Type A package (NDC# 69488-003-70). Distributed by: Advanced Accelerator Applications USA, Inc., Millburn, NJ 07041 |
Drugs
| Drug | Countries | |
|---|---|---|
| LUTATHERA | Austria, Estonia, Spain, France, Hong Kong, Croatia, Ireland, Israel, Italy, Japan, Lithuania, Poland, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.