LUTATHERA Solution for injection Ref.[109757] Active ingredients: Lutetium ¹⁷⁷Lu oxodotreotide
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
Lutetium Lu 177 dotatate binds to somatostatin receptors with highest affinity for subtype 2 somatostatin receptors (SSTR2). Upon binding to somatostatin receptor expressing cells, including malignant somatostatin receptor-positive tumors, the compound is internalized. The beta-minus emission from lutetium-177 induces cellular damage by formation of free radicals in somatostatin receptor-positive cells and in neighboring cells.
12.2. Pharmacodynamics
Lutetium Lu 177 dotatate exposure-response relationships and the time course of pharmacodynamics response are unknown.
Cardiac Electrophysiology
The ability of LUTATHERA to prolong the QTc interval at the recommended dose was assessed in an openlabel study in 20 patients with somatostatin receptor-positive midgut carcinoid tumors. No large changes in the mean QTc interval (i.e., >20 ms) were detected.
12.3. Pharmacokinetics
The pharmacokinetics (PK) of lutetium Lu 177 dotatate have been characterized in patients with progressive, somatostatin receptor-positive neuroendocrine tumors. T he mean blood exposure (area under the curve) of lutetium Lu 177 dotatate at the recommended dose is 41 ng.h/mL [coefficient of variation (CV) 36%]. The mean maximum blood concentration (Cmax) for lutetium Lu 177 dotatate is 10 ng/mL (CV 50%), which generally occurred at the end of the LUTATHERA infusion.
Distribution
The mean volume of distribution (Vz) for lutetium Lu 177 dotatate is 460 L (CV 54%).
The non-radioactive lutetium Lu 175 dotatate is 43% bound to human plasma proteins.
Within 4 hours after administration, lutetium Lu 177 dotatate distributes in kidneys, tumor lesions, liver, spleen, and, in some patients, pituitary gland and thyroid. The co-administration of amino acids reduced the median radiation dose to the kidneys by 47% (34% to 59%) and increased the mean beta-phase blood clearance of lutetium Lu 177 dotatate by 36%.
Elimination
The mean clearance (CL) is 4.5 L/h (CV 31%) and the mean terminal half-life is 71 (±28) hours for lutetium 177 dotatate.
Metabolism
Lutetium Lu 177 dotatate does not undergo hepatic metabolism.
Excretion
Lutetium Lu 177 dotatate is primarily eliminated renally with cumulative excretion of 44% within 5 hours, 58% within 24 hours, and 65% within 48 hours following LUTATHERA administration. Prolonged elimination of lutetium Lu 177 dotatate in the urine is expected; however, based on the half-life of lutetium-177 and terminal half-life of lutetium Lu 177 dotatate, greater than 99% of the administered radioactivity will be eliminated within 14 days after administration of LUTATHERA [see Warnings and Precautions (5.1)].
Special populations
Pediatric patients
There were no clinically relevant differences in exposure of lutetium Lu 177 dotatate in pediatric patients 12 years and older compared to that of adult patients.
Drug Interaction Studies
In Vitro Studies
CYP450 enzymes: The non-radioactive lutetium Lu 175 dotatate is not an inhibitor or inducer of cytochrome P450 (CYP) 1A2, 2B6, 2C9, 2C19 or 2D6 in vitro.
Transporters: The non-radioactive lutetium Lu 175 dotatate is not an inhibitor of P-glycoprotein, BCRP, OAT1, OAT3, OCT1, OCT2, OATP1B1, or OATP1B3 in vitro.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with lutetium Lu 177 dotatate; however, radiation is a carcinogen and mutagen.
No animal studies were conducted to determine the effects of lutetium Lu 177 dotatate on fertility.
13.2. Animal Toxicology and/or Pharmacology
The primary target organ in animal studies using the non-radioactive lutetium Lu 175 dotatate was the pancreas, a high SSTR2 expressing organ. Pancreatic acinar apoptosis occurred at lutetium Lu 175 dotatate doses ≥5 mg/kg in repeat dose toxicology studies in rats. Pancreatic acinar cell atrophy also occurred in repeat dose toxicology studies in dogs at doses ≥500 mcg/kg. These findings were consistent with high uptake of the radiolabeled peptide in the pancreas in animal biodistribution studies.
14. Clinical Studies
14.1 Progressive, Well-Differentiated Advanced or Metastatic Somatostatin Receptor-Positive Midgut Carcinoid Tumors
The efficacy of LUTATHERA in patients with progressive, well-differentiated, locally advanced/inoperable or metastatic somatostatin receptor-positive midgut carcinoid tumors was established in NETTER-1 (NCT01578239), a randomized, multicenter, open-label, active-controlled trial. Key eligibility criteria included Ki67 index ≤20%, Karnofsky performance status ≥60, confirmed presence of somatostatin receptors on all lesions (OctreoScan uptake ≥ normal liver), creatinine clearance ≥50 mL/min, no prior treatment with peptide receptor radionuclide therapy (PRRT), and no prior external beam radiation therapy to more than 25% of the bone marrow.
At the time of the primary analysis, 229 patients were randomized (1:1) to receive either LUTATHERA 7.4 GBq (200 mCi) every 8 weeks (±1 week) for up to 4 administrations (maximum cumulative dose of 29.6 GBq) or high-dose long-acting octreotide (defined as 60 mg by intramuscular injection every 4 weeks). Patients in the LUTATHERA arm also received long-acting octreotide 30 mg as an intramuscular injection 4 to 24 hours after each LUTATHERA dose and every 4 weeks after completion of LUTATHERA treatment until disease progression or until week 76 of the study. Patients in both arms could receive short-acting octreotide for symptom management; however, short-acting octreotide was withheld at least 24 hours before each LUTATHERA dose. Randomization was stratified by OctreoScan tumor uptake score (Grade 2, 3 or 4) and the length of time that patients had been on the most recent constant dose of octreotide prior to randomization (≤6 or >6 months). The major efficacy outcome measure was progression-free survival (PFS) as determined by a blinded independent review committee (IRC) per RECIST v1.1. Additional efficacy outcome measures were overall response rate (ORR) by IRC, duration of response (DoR) by IRC, and overall survival (OS).
Demographic and baseline disease characteristics were balanced between the treatment arms. Of the 229 patients, 82% were White, 4% were Black, 3% were Hispanic or Latino, 0.4% were Asian, 0.4% were Other, and 9% were not reported. The median age was 64 years (28 to 87 years); 51% were male, 74% had an ileal primary, and 96% had metastatic disease in the liver. The median Karnofsky performance score was 90 (60 to 100), 74% received a constant dose of octreotide for >6 months and 12% received prior treatment with everolimus. Sixty-nine percent of patients had Ki67 expression in ≤ 2% of tumor cells, 77% had CgA >2 times the upper limit of normal (ULN), 65% had 5-HIAA >2 times ULN, and 65% had alkaline phosphatase ≤ ULN.
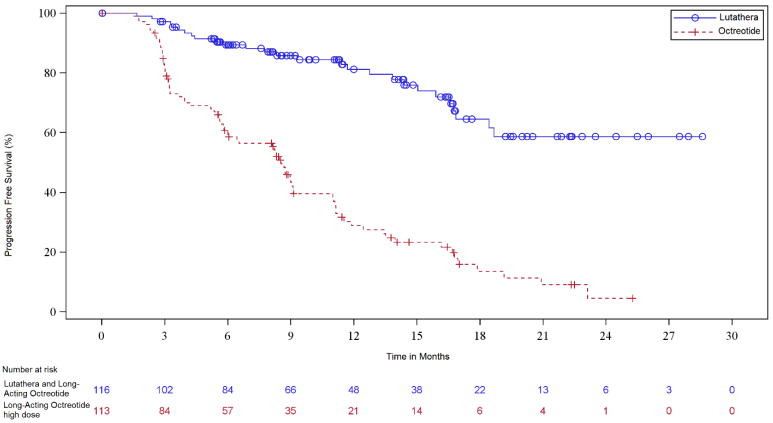
At the time of the final OS analysis, which occurred 66 months after the primary PFS analysis, 117 patients were randomized to the LUTATHERA arm and 114 patients were randomized to the octreotide arm. In the final OS analysis, there was no statistically significant difference in OS between the two treatment arms. Efficacy results for NETTER-1 are presented in Table 9 and Figure 1.
Table 9. Efficacy Results in NETTER-1:
| LUTATHERA with Longacting Octreotide (30 mg) N=116 | Long-acting Octreotide (60 mg) N=113 | |
|---|---|---|
| PFS by IRC | ||
| Events (%) | 27 (23%) | 78 (69%) |
| Progressive disease, n (%) | 15 (13%) | 61 (54%) |
| Death, n (%) | 12 (10%) | 17 (15%) |
| Median in months (95% CI) | NR (18.4, NE) | 8.5 (6.0, 9.1) |
| Hazard ratioa (95% CI) | 0.21 (0.13, 0.32) | |
| p-valueb | <0.0001 | |
| ORR by IRC | ||
| ORR, % (95% CI) | 13% (7%, 19%) | 4% (0.1%, 7%) |
| Complete response rate, n (%) | 1 (1%) | 0 |
| Partial response rate, n (%) | 14 (12%) | 4 (4%) |
| p-valuec | 0.0148 | |
Duration of response, median in months (95% CI) NR (2.8, NE) 1.9 (1.9, NE)
Abbreviations: CI, confidence interval; IRC, independent radiology committee; NE, not evaluable; NR, not reached; ORR, overall response rate; PFS, progression-free survival.
a Hazard ratio based on the unstratified Cox model.
b Unstratified log rank test.
c Fisher’s exact test.
Figure 1. Kaplan-Meier Curves for Progression-Free Survival in NETTER-1:
14.2 Somatostatin Receptor-Positive Gastroenteropancreatic Neuroendocrine Tumors
The efficacy of LUTATHERA in patients with foregut, midgut, and hindgut gastroenteropancreatic neuroendocrine tumors (GEP-NETs) was assessed in 360 patients in the ERASMUS study. In ERASMUS, LUTATHERA was initially provided as expanded access under a general peptide receptor radionuclide therapy protocol at a single site in the Netherlands. A subsequent LUTATHERA-specific protocol written eight years after study initiation did not describe a specific sample size or hypothesis testing plan but allowed for retrospective data collection. A total of 1214 patients received LUTATHERA in ERASMUS, of whom 578 patients had baseline tumor assessments. Of the 578 patients, 360 (62%) had GEP-NETs and long-term follow-up. Of these 360 patients, 145 (40%) had their tumors prospectively evaluated according to RECIST criteria. LUTATHERA 7.4 GBq (200 mCi) was administered every 6 to 13 weeks for up to 4 doses concurrently with the recommended amino acid solution. The major efficacy outcome was investigator-assessed ORR. The median age in the efficacy subset was 60 years (30 to 85 years), 51% were male, 71% had a baseline Karnofsky performance status ≥90, 51% had progressed within 12 months of treatment, and 7% had received prior chemotherapy. Fifty-two percent (52%) of patients received a concomitant somatostatin analog. The median dose of LUTATHERA was 29.6 GBq (800 mCi). The investigator-assessed ORR was 17% (95% CI: 13, 21) based on an analysis that required responders to have had prospective response assessments according to RECIST criteria. Three complete responses were observed (<1%). Median DoR in the 60 responding patients was 35 months (95% CI: 17, 38).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.