FINZALA Chewable tablet, Tablet Ref.[109375] Active ingredients: 17 alpha-Ethinylestradiol Noradrenaline
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
Finzala (norethindrone acetate and ethinyl estradiol tablets USP, 1 mg/20 mcg and ferrous fumarate tablets) provide an oral contraceptive regimen consisting of 24 white active chewable tablets that contain the active ingredients, followed by 4 brown non-hormonal placebo tablets as specified below:
- 24 white, round, flat-faced, beveled-edge, unscored tablets each containing 1 mg norethindrone acetate, USP and 20 mcg ethinyl estradiol, USP.
- 4 brown, round, flat-faced, beveled-edge, unscored tablets each containing 75 mg ferrous fumarate, USP.
Each white active chewable tablet also contains the following inactive ingredients: acacia, confectioner’s sugar, lactose monohydrate, magnesium stearate, maltodextrin, pregelatinized corn starch, silicon dioxide, spearmint oil, sucralose, and talc.
Each brown placebo tablet contains ferrous fumarate USP, magnesium stearate, maltodextrin, mannitol, microcrystalline cellulose, povidone, sodium starch glycolate type A, silicon dioxide, spearmint oil, and sucralose. The ferrous fumarate tablets do not serve any therapeutic purpose. Ferrous fumarate tablets are not USP for dissolution and assay.
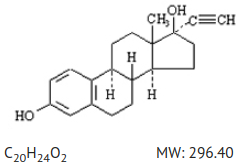
The structural formula of ethinyl estradiol, USP is:
The chemical name of ethinyl estradiol, USP is [19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol, (17α)-].
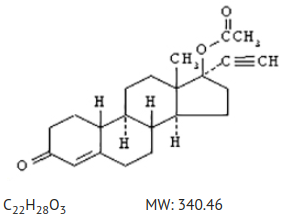
The structural formula of norethindrone acetate, USP is:
The chemical name of norethindrone acetate, USP is [19-Norpregn-4-en-20-yn-3-one, 17-(acetyloxy), (17α)].
| Dosage Forms and Strengths |
|---|
|
Finzala (norethindrone acetate and ethinyl estradiol tablets USP, 1 mg/20 mcg and ferrous fumarate tablets) is available in blister packs. Each blister pack contains 28 tablets in the following order:
|
| How Supplied |
|---|
|
Finzala (norethindrone acetate and ethinyl estradiol tablets USP, 1 mg/20 mcg and ferrous fumarate tablets*) is available in blister cards (dispensers) containing 28 tablets: NDC 0093-8210-62 Cartons of 3 blister cards (dispensers) Each blister card contains 28 tablets in the following order:
*Ferrous fumarate tablets are not USP for dissolution and assay. Manufactured By: Teva Pharmaceuticals USA, Inc., Parsippany, NJ 07054. |
Drugs
| Drug | Countries | |
|---|---|---|
| FINZALA | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.