FINZALA Chewable tablet, Tablet Ref.[109375] Active ingredients: 17 alpha-Ethinylestradiol Noradrenaline
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
CHCs lower the risk of becoming pregnant primarily by suppressing ovulation.No specific pharmacodynamic studies were conducted with Finzala.
12.2. Pharmacodynamics
No specific pharmacodynamic studies were conducted with Finzala.
12.3. Pharmacokinetics
Absorption
In a single-dose, two-way, crossover clinical study conducted in 35 healthy, non-smoking premenopausal women under fasting condition, norethindrone acetate and ethinyl estradiol tablets chewed and swallowed were bioequivalent to norethindrone acetate 1 mg/ethinyl estradiol 20 mcg tablets (24-day regimen tablets) swallowed whole based on the exposure (AUC) and peak concentration (Cmax) of norethindrone and ethinyl estradiol.
Norethindrone acetate appears to be completely and rapidly deacetylated to norethindrone after oral administration, because the disposition of norethindrone acetate is indistinguishable from that of orally administered norethindrone. Norethindrone acetate and ethinyl estradiol are absorbed from Finzala tablets (chewed and swallowed), with maximum plasma concentrations of norethindrone and ethinyl estradiol occurring at 1.0 hr (range: 0.7 to 2.5 hrs) and 1.3 hr (range: 1 to 2.5 hrs) post-dose, respectively. Both are subject to first-pass metabolism after oral dosing, resulting in an absolute bioavailability of approximately 64% for norethindrone and 43% for ethinyl estradiol.
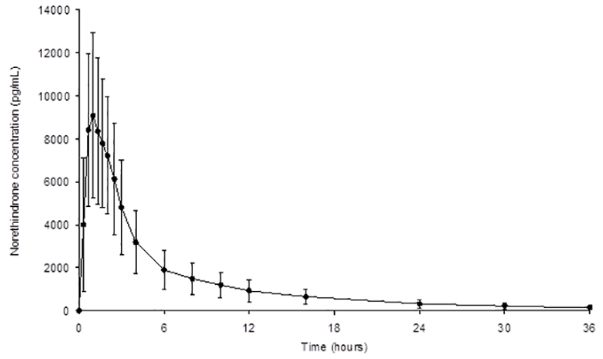
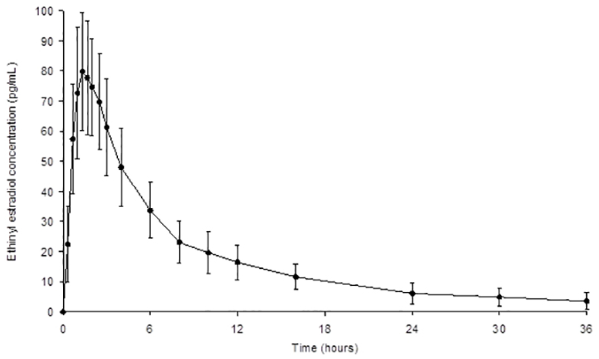
The plasma norethindrone and ethinyl estradiol pharmacokinetics following single-dose administrations of norethindrone acetate and ethinyl estradiol tablets (chewed and swallowed) in 35 healthy female subjects are provided in Figures 2 and 3, and Table 1.
Following multiple-dose administration of norethindrone acetate/ethinyl estradiol tablets (swallowed whole) in 17 healthy female subjects, mean maximum concentrations of norethindrone and ethinyl estradiol were increased by 95% and 27%, respectively, as compared to single-dose administration. Mean norethindrone and ethinyl estradiol exposures (AUC values) were increased by 164% and 51% respectively, as compared to single-dose administration of norethindrone acetate/ethinyl estradiol tablets.
Steady-state with respect to norethindrone was reached by Day 17 and steady-state with respect to ethinyl estradiol was reached by Day 13.
Mean SHBG concentrations were increased by 150% from baseline (57.5 nmol/L) to 144 nmol/L at steady-state.
Figure 2. Mean (± Standard Deviation) Plasma Norethindrone Concentration-Time Profile Following Single-Dose Oral Administration of Norethindrone Acetate and Ethinyl Estradiol Tablets (chewed and swallowed) to Healthy Female Volunteers under Fasting Conditions (n = 35):
Figure 3. Mean (± Standard Deviation) Plasma Ethinyl Estradiol Concentration-Time Profile Following Single-Dose Oral Administration of Norethindrone Acetate and Ethinyl Estradiol Tablets (chewed and swallowed) to Healthy Female Volunteers under Fasting Conditions (n = 35):
Table 1. Summary of Norethindrone (NE) and Ethinyl Estradiol (EE) Pharmacokinetics Following Single-Dose Oral Administration of Norethindrone Acetate and Ethinyl Estradiol Tablets (chewed and swallowed) to Healthy Female Volunteers Under Fasting Conditions (n = 35):
| Analyte | Arithmetic Mean a (% CV) by Pharmacokinetic Parameter | ||||
| Cmax(pg/mL) | tmax(hr) | AUC(0±tldc)(pg/mL•h) | AUC(0±inf)(pg/mL•h) | t½(hr) | |
| NE | 10200(36) | 1.03(0.67 to 2.50) | 48620(40) | 49250(40) | 8.58 |
| EE | 84.7(24) | 1.33(1.00 to 2.50) | 677.5(33) | 741.6(33) | 9.68 |
| Cmax = Maximum plasma concentration | |||||
| tmax = Time of Cmax | |||||
| AUC(0±tldc) = Area under plasma concentration versus time curve from 0 to tldc, the time of last determinable concentration | |||||
| AUC(0±inf) = Area under the plasma concentration versus time curve from time 0 to infinity | |||||
| t½ = Terminal phase half-life | |||||
| % CV = Coefficient of Variation (%) | |||||
a The harmonic mean (0.693/mean terminal phase rate constant) is reported for t½, and the median (range) is reported for tmax
Food Effect
Finzala tablets may be administered without regard to meals.
A single-dose administration of norethindrone acetate/ethinyl estradiol tablets with food decreased the maximum concentration of norethindrone by 51% and increased the extent of absorption by 15% and decreased the maximum concentration of ethinyl estradiol by 51% but not the extent of absorption.
Distribution
Volume of distribution of norethindrone and ethinyl estradiol ranges from 2 to 4 L/kg. Plasma protein binding of both steroids is extensive (greater than 95%); norethindrone binds to both albumin and SHBG, whereas ethinyl estradiol binds only to albumin. Although ethinyl estradiol does not bind to SHBG, it induces SHBG synthesis.
Metabolism
Norethindrone undergoes extensive biotransformation, primarily via reduction, followed by sulfate and glucuronide conjugation. The majority of metabolites in the circulation are sulfates, with glucuronides accounting for most of the urinary metabolites.
Ethinyl estradiol is also extensively metabolized, both by oxidation and by conjugation with sulfate and glucuronide. Sulfates are the major circulating conjugates of ethinyl estradiol and glucuronides predominate in urine. The primary oxidative metabolite is 2-hydroxy ethinyl estradiol, formed by the CYP3A4 isoform of cytochrome P450. Part of the first-pass metabolism of ethinyl estradiol is believed to occur in gastrointestinal mucosa. Ethinyl estradiol may undergo enterohepatic circulation.
Excretion
Norethindrone and ethinyl estradiol are excreted in both urine and feces, primarily as metabolites. Plasma clearance values for norethindrone and ethinyl estradiol are similar (approximately 0.4 L/hr/kg). Steady-state elimination half-lives of norethindrone and ethinyl estradiol following administration of norethindrone acetate/ethinyl estradiol tablets are approximately 8 hours and 14 hours, respectively.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
[See Warnings and Precautions (5.2, 5.11)].
14. Clinical Studies
The data presented in Section 14 are from a clinical trial conducted with a 24-day regimen of norethindrone acetate 1 mg/ethinyl estradiol 20 mcg tablets. Finzala tablets are bioequivalent to these norethindrone acetate/ethinyl estradiol tablets.
In a clinical study, 743 women 18 to 45 years of age were studied to assess the efficacy of norethindrone acetate/ethinyl estradiol tablets, for up to six 28-day cycles providing a total of 3,823 treatment-cycles of exposure. The racial demographic of all enrolled women was: 70% Caucasian, 16% African-American, 10% Hispanic, 2% Asian and 2% Other. Women with body mass index (BMI) greater than 35 kg/m 2 were excluded from the study. The weight range for those women treated was 90 to 260 pounds, with a mean weight of 147 pounds. Among the women in the study, about 40% had not used hormonal contraception immediately prior to enrolling in this study.
A total of 583 women completed 6 cycles of treatment. There were a total of 5 on-treatment pregnancies in 3,565 treatment cycles during which no backup contraception was used. The Pearl Index for norethindrone acetate and ethinyl estradiol tablets was 1.82 (95% confidence interval 0.59 to 4.25).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.