POSLUMA Solution for injection Ref.[109999] Active ingredients: Flotufolastat ¹⁸F
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
11.1 Chemical Characteristics
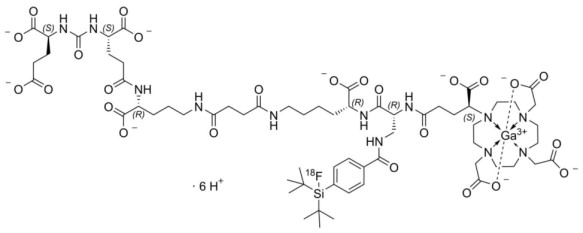
POSLUMA (flotufolastat F 18) injection is a radioactive diagnostic agent for intravenous use. The active ingredient of POSLUMA is flotufolastat F 18 gallium, of which the molecular structure includes a DOTAGA complex with nonradioactive gallium. Radioactive fluorine-18 is covalently bound to silicon.
Chemically, flotufolastat F 18 gallium is gallate(6-), [(4 S,8 S,13 R,27 R,30 R,35 S)35[4,10-bis[(carboxy-k O)methyl]7(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl-k N 1,k N 4,k N 7,k N 10]30[[[4-[bis(1,1-dimethylethyl)fluoro- 18F-silyl]benzoyl]amino]methyl]-1,36-dihydroxy-1,6,11,18,21,29,32,36-octaoxo-5,7,12,17,22,28,31-heptaazahexatriacontane-4,8,13,27-tetracarboxylato(9-)]-, hydrogen (1:6).
The molecular weight is 1537.3 g/mol and the structural formula is:
POSLUMA is a sterile, non-pyrogenic, clear, colorless, and isotonic solution. Each mL contains up to 20 mcg of flotufolastat gallium, up to 5,846 MBq (158 mCi) as flotufolastat F 18 gallium at end of synthesis, and the following inactive ingredients: not more than 10% (v/v) alcohol, 1.9 mg anhydrous citric acid, 7.2 mg sodium chloride, and 0.75 mg sodium hydroxide to adjust pH between 4 and 6. POSLUMA contains no preservative.
11.2 Physical Characteristics
POSLUMA contains fluorine-18 (F 18) which is a cyclotron produced radionuclide that decays by positron emission (β+ decay, 96.7%) and orbital electron capture (3.3%) to stable oxygen-18 with a physical half-life of 109.8 minutes (Table 3). The principal photons useful for diagnostic imaging are the coincident pair of 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 4).
Table 3. Physical Decay Chart for Fluorine-18:
| Minutes | Fraction Remaining |
|---|---|
| 0 | 1 |
| 15 | 0.909 |
| 30 | 0.826 |
| 60 | 0.683 |
| 110 | 0.5 |
| 220 | 0.25 |
Table 4. Principal Radiation Produced from Decay of Fluorine-18:
| Energy (keV) | Abundance (%) | |
|---|---|---|
| Positron | 249.8 | 96.7 |
| Gamma | 511 | 193.5 |
11.3 External Radiation
The point source air-kerma coefficient for F 18 is 3.75 × 10 -17 Gy m2/(Bq s). The first half-value thickness of lead (Pb) for F 18 gamma rays is approximately 6 mm. The relative reduction of radiation emitted by F 18 that results from various thicknesses of lead shielding is shown in Table 5. The use of 8 cm of Pb will decrease the radiation transmission (i.e., exposure) by a factor of about 10,000.
Table 5. Radiation Attenuation of 511 keV Gamma Rays by Lead Shielding:
| Shield Thickness cm of Lead (Pb) | Coefficient of Attenuation |
|---|---|
| 0.6 | 0.5 |
| 2 | 0.1 |
| 4 | 0.01 |
| 6 | 0.001 |
| 8 | 0.0001 |
| Dosage Forms and Strengths |
|---|
|
Injection: 296 MBq/mL to 5,846 MBq/mL (8 mCi/mL to 158 mCi/mL) as flotufolastat F 18 gallium in approximately 25 mL at end of synthesis supplied as a clear, colorless solution in a multiple-dose vial. |
| How Supplied |
|---|
|
POSLUMA injection is supplied as a clear, colorless solution in a multiple-dose glass vial (NDC 69932-002-50) containing 296 MBq/mL to 5,846 MBq/mL (8 mCi/mL to 158 mCi/mL) as flotufolastat F 18 gallium in approximately 25 mL at end of synthesis. Marketed by Blue Earth Diagnostics Ltd., Oxford, OX4 4GA, UK |
Drugs
| Drug | Countries | |
|---|---|---|
| POSLUMA | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.