SIGNIFOR LAR Suspension for injection Ref.[27986] Active ingredients: Pasireotide
Source: Health Products and Food Branch (CA) Revision Year: 2020
Indications and clinical use
- SIGNIFOR LAR (pasireotide) is indicated for the treatment of adult patients with acromegaly for whom surgery is not an option or has not been curative.
- SIGNIFOR LAR (pasireotide) is indicated for the treatment of adult patients with Cushing’s disease for whom surgery is not an option or for whom surgery has failed, as long as clinical benefit or normalization of urinary free cortisol (UFC) (or >50% decrease in UFC) are derived (see CLINICAL TRIALS, Cushing’s disease).
The 60 mg dose is only to be used for the treatment of acromegaly (see DOSAGE AND ADMINISTRATION).
SIGNIFOR LAR should be prescribed and supervised by a qualified physician.
Geriatrics (≥65 years of age)
There are limited data on the use of SIGNIFOR LAR in acromegaly patients and very limited data in Cushing’s disease patients older than 65 years. Clinical studies of SIGNIFOR LAR did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients (see WARNINGS AND PRECAUTIONS, Special Populations, Geriatrics).
Pediatrics (<18 years of age)
SIGNIFOR LAR should not be used in pediatric patients. There are no clinical data available in patients under 18 years of age (see WARNINGS AND PRECAUTIONS, Special Populations, Pediatrics).
Dosage and administration
Recommended Dose and Dosage Adjustment
Acromegaly
The recommended initial dose of SIGNIFOR LAR for the treatment of acromegaly is 40 mg administered by deep intramuscular injection every 4 weeks (q28d).
The dose may be increased to a maximum of 60 mg for patients whose GH and/or IGF-1 levels are not fully controlled after 3 months of treatment with SIGNIFOR LAR at 40 mg and who tolerate this dose (see ADVERSE REACTIONS, Acromegaly and CLINICAL TRIALS, Acromegaly).
Management of suspected adverse reactions or over response to treatment (age and sex adjusted IGF-1 < LLN) may require dose reduction of SIGNIFOR LAR. The dose may be decreased, either temporarily or permanently, by 20 mg decrements. Efficacy should be monitored closely as there are limited data with the use of the 20 mg dose.
Cushing’s disease
The recommended initial dose of SIGNIFOR LAR for the treatment of Cushing’s disease is 10 mg administered by deep intramuscular injection every 4 weeks (q28d).
Patients should be evaluated for treatment response after the first month of treatment and periodically thereafter (see WARNINGS AND PRECAUTIONS – MONITORING AND LABORATORY TESTS, Cushing’s disease). The dose may be titrated every 2 to 4 months based on response and tolerability (see ADVERSE REACTIONS, Cushing’s disease and CLINICAL TRIALS, Cushing’s disease). The maximum dose of SIGNIFOR LAR in Cushing’s disease is 40 mg q28d (see INDICATIONS AND CLINICAL USE). If no clinical benefit is observed at the maximum tolerated dose, the patient should be considered for discontinuation.
Management of suspected adverse reactions or over-response to treatment (e.g., cortisol levels less than the lower limit of normal range or in the low part of the normal range in patients with symptoms suggestive of adrenal insufficiency) may require dose reduction to the previous tolerated dose, interruption, or discontinuation of SIGNIFOR LAR. For patients treated with 10 mg once every 28 days, the dose may be either interrupted or discontinued (see WARNINGS AND PRECAUTIONS – Endocrine and Metabolism, Hypocortisolism and MONITORING AND LABORATORY TESTS, Hypocortisolism).
Switch from subcutaneous to intramuscular formulation in Cushing’s disease
There are no clinical data available on switching from the subcutaneous to the intramuscular pasireotide formulation. If such a switch would be considered, the recommended initial dose for the treatment of Cushing’s disease is 10 mg of SIGNIFOR LAR administered by deep intramuscular injection once every 4 weeks. The patient should be monitored for response and tolerability, and further dose adjustment, interruption, or discontinuation may be required.
Recommended Baseline Evaluations Prior to Initiation of SIGNIFOR LAR
Prior to the start of SIGNIFOR LAR, patients should have the following baseline evaluations (see WARNINGS AND PRECAUTIONS):
- Fasting Plasma Glucose
- Hemoglobin A1c
- Liver tests
- Electrocardiogram
- Gallbladder ultrasound
SIGNIFOR LAR is contraindicated in patients with uncontrolled diabetes mellitus (see CONTRAINDICATIONS).
Special populations
Renal impairment
No dose adjustment is required in patients with mild to moderate renal impairment. In a clinical study of single dose pasireotide s.c. 900 µg, in patients with various degrees of renal impairment, grade 3 and grade 4 increases in amylase, lipase, and uric acid and grade 3 decreases in hemoglobin were observed in subjects with severe renal impairment and ESRD. SIGNIFOR LAR should be used with caution in patients with severe renal impairment and ESRD (see WARNINGS AND PRECAUTIONS – Renal, WARNINGS AND PRECAUTIONS – Monitoring and Laboratory Tests, DOSAGE AND ADMINISTRATION, and ACTION AND CLINICAL PHARMACOLOGY).
Hepatic impairment
Dose adjustment is not required in patients with mildly impaired hepatic function (Child-Pugh A). SIGNIFOR LAR is contraindicated in patients with moderate or severe hepatic impairment (Child Pugh B or C) (see CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS).
Pediatric patients (<18 years of age)
The safety and efficacy of SIGNIFOR LAR in patients under 18 years of age have not been established. SIGNIFOR LAR should not be used in pediatric patients (see INDICATIONS AND CLINICAL USE and WARNINGS AND PRECAUTIONS – Special Populations, Pediatrics).
Geriatric patients (≥65 years of age)
There are limited data on the use of SIGNIFOR LAR in patients older than 65 years (see INDICATIONS AND CLINICAL USE and WARNINGS AND PRECAUTIONS – Special Populations, Geriatrics). Generally, dose selection for elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Missed Dose
If a dose of SIGNIFOR LAR is missed, the injection should be administered as soon as possible and next injection should be planned after 4 weeks to reassume normal schedule every 4 weeks.
Administration
SIGNIFOR LAR should only be administered by deep intramuscular injection by a trained health care professional. SIGNIFOR LAR suspension must only be prepared immediately before administration. The site of repeat intramuscular injections should be alternated between the left and right gluteal muscle.
Reconstitution
PARENTERAL PRODUCT FOR DEEP INTRAMUSCULAR INJECTION ONLY
ATTENTION: There are 2 critical steps in the reconstitution of SIGNIFOR LAR. Not following them could result in failure to deliver the drug appropriately.
- The injection kit must reach room temperature. Remove the injection kit from the fridge and let the kit stand at room temperature for a minimum of 30 minutes before reconstitution, but do not exceed 24 hours.
- After adding the diluent solution, shake the vial moderately in a horizontal direction for a minimum of 30 seconds until uniform suspension is formed.
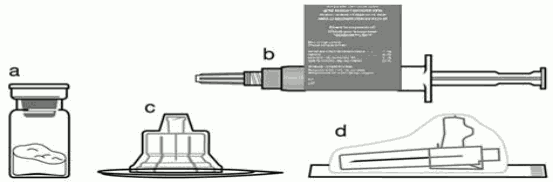
Included in the injection kit:
a. One vial containing SIGNIFOR LAR powder 20mg, 40mg, or 60 mg pasireotide for injectable suspension
b. One prefilled syringe containing the diluent solution for reconstitution (showing the peel-off outer syringe label)
c. One vial adapter for drug product reconstitution
d. One safety injection needle (20G x 1.5")
e. One instruction booklet
f. The package insert
Follow the instructions below carefully to ensure proper reconstitution of SIGNIFOR LAR before deep intramuscular injection.
SIGNIFOR LAR suspension must only be prepared immediately before administration.
SIGNIFOR LAR should only be administered by a trained health care professional.
| Step 1. Remove the SIGNIFOR LAR injection kit from refrigerated storage. ATTENTION: It is essential to start the reconstitution process only after the injection kit reaches room temperature. Let the kit stand at room temperature for a minimum of 30 minutes before reconstitution, but do not exceed 24 hours. Note: The injection kit can be re-refrigerated if needed but should not be refrigerated after reconstitution. | 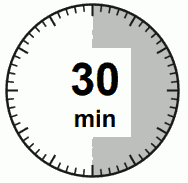 |
| Step 2. Remove the plastic cap from the vial and clean the rubber stopper of the vial with an alcohol wipe. Place the package on a clean and flat surface. Remove the lid film of the vial adapter packaging, but do NOT remove the vial adapter from its packaging. Holding the vial adapter packaging, position the vial adapter on top of the vial and push it fully down so that it snaps in place, confirmed by an audible “click”. Hold the vial firmly and lift the packaging off the vial adapter with a vertical movement. | 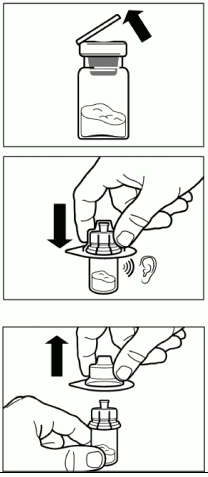 |
| Step 3. Remove the cap from the syringe prefilled with diluent solution and screw the syringe clockwise onto the vial adapter. Slowly push the plunger all the way down to transfer all the diluent solution in the vial. | 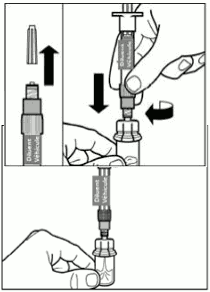 |
| Step 4. ATTENTION: Keep the plunger pressed and shake the vial moderately in a horizontal direction for a minimum of 30 seconds so that the powder is completely suspended. Repeat moderate shaking for another 30 seconds if the powder is not completely suspended. | 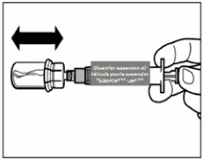 |
| Step 5. Turn syringe and vial upside down, slowly pull the plunger back and draw the entire content from the vial into the syringe. Unscrew the syringe counterclockwise from the vial adapter. Ensure that the tip of the syringe remains sterile. | 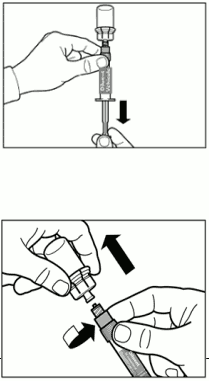 |
| Step 6. The product in the syringe now consists of reconstituted SIGNIFOR LAR for Injectable Suspension. The suspension should be milky, slightly yellowish to yellowish and homogeneous. To avoid confusion, peel off the outer syringe label which corresponds only with the diluent. It is no longer a correct representation of the current contents of the syringe. | 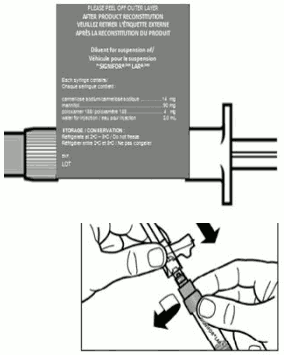 |
| Step 7. Screw the safety injection needle onto the syringe. Pull the protective cover straight off the needle. To avoid sedimentation, you may gently shake the syringe to maintain a uniform suspension. Gently tap the syringe to remove any visible bubbles and expel them from the syringe. The reconstituted SIGNIFOR LAR is now ready for immediate administration. | 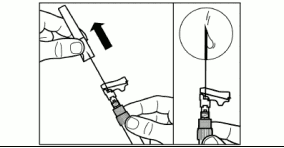 |
| Step 8. SIGNIFOR LAR must be given only by deep intramuscular injection; NEVER intravenously. Prepare the injection site with a disinfectant. Insert the needle fully into the left or right gluteus at a 90° angle to the skin. Slowly pull back the plunger to check that no blood vessel has been penetrated (reposition if a blood vessel has been penetrated). Slowly depress the plunger until the syringe is empty. Withdraw the needle from the injection site and activate the safety guard (as shown in Step 9). | 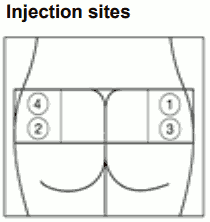 |
| Step 9. Activate the safety guard over the needle, in one of the 2 methods shown: * either press the hinged section of the safety guard down onto a hard surface (figure A), * or push the hinge forward with your finger (figure B). An audible “click” confirms proper activation. Dispose of syringe immediately in a sharps container. | 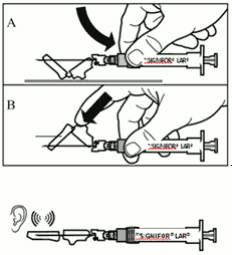 |
Parenteral Products
| Vial Size | Volume of Diluent to be Added to Vial | Approximate Available Volume | Nominal Concentration per mL |
|---|---|---|---|
| 6 mL | 2 mL | 2 mL | 5 mg/mL (10 mg strength) 10 mg/mL (20 mg strength) 15 mg/mL (30 mg strength) 20 mg/mL (40 mg strength) 30 mg/mL (60 mg strength) |
Overdosage
In the event of overdosage, it is recommended that appropriate supportive treatment be initiated, as dictated by the patient’s clinical status, until resolution of the symptoms. Electrocardiogram monitoring is recommended.
For management of a suspected drug overdose, contact your regional Poison Control Centre.
Storage and stability
Store at 2 to 8°C. Do not freeze.
Prior to reconstitution, the injection kit should be removed from the fridge and equilibrated to room temperature for a minimum of 30 minutes and maximum of 24 hours.
The suspension should be administered immediately after reconstitution.
SIGNIFOR LAR (pasireotide as pamoate) must be kept out of the reach and sight of children.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.