Ibuprofen
Chemical formula: C₁₃H₁₈O₂ Molecular mass: 206.281 g/mol PubChem compound: 3672
Mechanism of action
Ibuprofen is a propionic acid derivative with analgesic, anti-inflammatory and anti-pyretic activity. The drug's therapeutic effects as an NSAID is thought to result from its inhibitory effect on the enzyme cyclo-oxygenase, which results in a marked reduction in prostaglandin synthesis.
Pharmacodynamic properties
Experimental data suggest that ibuprofen may competitively inhibit the effect of low dose aspirin on platelet aggregation when they are dosed concomitantly. Some pharmacodynamic studies show that when single doses of ibuprofen 400mg were taken within 8 hours before or within 30 minutes after immediate release aspirin dosing (81mg), a decreased effect of aspirin on the formation of thromboxane or platelet aggregation occurred. Although there are uncertainties regarding extrapolation of these data to the clinical situation, the possibility that regular, long-term use of ibuprofen may reduce the cardioprotective effect of low-dose acetylsalicylic acid cannot be excluded. No clinically relevant effect is considered to be likely for occasional ibuprofen use.
In the form of a medicated plaster, which locally delivers ibuprofen continuously at the site of pain over the 24 hours of application, it has topical anti-inflammatory and analgesic activity. Pooled data from two clinical efficacy and safety studies in adults with acute soft tissue injuries showed that when applied once every 24h, the medicated plaster provided long lasting relief, with a statistically significant decrease in pain on movement compared with a placebo plaster from 2hrs post first dose and every subsequent time point over 5 days. Analysis of tenderness at the injured site also showed a significant difference compared with placebo at 24 and 120 hours following use.
Pharmacokinetic properties
Oral administration
Absorption
Ibuprofen is rapidly absorbed from the gastrointestinal tract with a bioavailability of 80-90%. Peak serum concentrations occur one to two hours after administration. If administered with food, peak serum concentrations are lower and achieved more slowly than when taken on an empty stomach Food does not affect markedly total bioavailability.
Distribution
Ibuprofen is extensively bound to plasma proteins (99%). Ibuprofen has a small volume of distribution being about 0.12-0.2 L/kg in adults.
Biotransformation
Ibuprofen is rapidly metabolized in the liver through cytochrome P450, preferentially CYP2C9, to two primary inactive metabolites, 2-hydroxyibuprofen and 3-carboxyibuprofen. Following oral ingestion of the drug, slightly less than 90% of an oral dose of ibuprofen can be accounted for in the urine as oxidative metabolites and their glucuronic conjugates. Very little ibuprofen is excreted unchanged in the urine.
Elimination
Excretion by the kidney is both rapid and complete. The elimination half-life is approximately 2 hours. The excretion of ibuprofen is virtually complete 24 hours after the last dose.
Special populations
Elderly
Given that no renal impairment exists, there are only small, clinically insignificant differences in the pharmacokinetic profile and urinary excretion between young and elderly.
Children
The systemic exposure of ibuprofen following weight adjusted therapeutic dosage (5mg/kg to 10 mg/kg bodyweight) in children aged 1 year or over, appears similar to that in adults.
Children 3 months to 2.5 years appeared to have a higher volume of distribution (L/kg) and clearance (L/kg/h) of ibuprofen than did children >2.5 to 12 years of age.
Renal impairment
For patients with mild renal impairment increased unbound (S)-ibuprofen, higher AUC values for (S)-ibuprofen and increased enantiomeric AUC (S/R) ratios as compared with healthy controls have been reported.
In end-stage renal disease patients receiving dialysis the mean free fraction of ibuprofen was about 3% compared with about 1% in healthy volunteers. Severe impairment of renal function may result in accumulation of ibuprofen metabolites. The significance of this effect is unknown. The metabolites can be removed by haemodialysis.
Hepatic impairment
Alcoholic liver disease with mild to moderate hepatic impairment did not result in substantially altered pharmacokinetic parameters.
In cirrhotic patients with moderate hepatic impairment (Child Pugh's score 6-10) treated with racemic ibuprofen an average 2-fold prolongation of the half-life was observed and the enantiomeric AUC ratio (S/R) was significantly lower compared to healthy controls suggesting an impairment of metabolic inversion of (R)-ibuprofen to the active (S)-enantiomer.
IV administration
Distribution
Although a great variability is observed in the premature population, peak plasma concentrations are measured around 35-40 mg/l after the initial loading dose of 10 mg/kg as well as after the last maintenance dose, whatever gestational and postnatal age. Residual concentrations are around 10-15 mg/l 24 hours after the last dose of 5 mg/kg.
Plasma concentrations of the S-enantiomer are much higher than those of the R-enantiomer, which reflects a rapid chiral inversion of the R- to the S-form in a proportion similar to adults (about 60%).
The apparent volume of distribution is on average 200 ml/kg (62 to 350 according to various studies). The central volume of distribution may depend on the status of the ductus and decrease as the ductus closes.
In vitro studies suggest that, similarly to other NSAIDs, ibuprofen is highly bound to plasma albumin, although this seems to be significantly lower (95%) compared with adult plasma (99%). Ibuprofen competes with bilirubin for albumin binding in newborn infant serum and, as a consequence, the free fraction of bilirubin may be increased at high ibuprofen concentrations.
Elimination
Elimination rate is markedly lower than in older children and adults, with an elimination half-life estimated at approximately 30 hours (16–43). The clearance of both enantiomers increases with gestational age, at least in the range of 24 to 28 weeks.
PK-PD relationship
In preterm newborns ibuprofen significantly reduced plasma concentrations of prostaglandins and their metabolites, particularly PGE2 and 6-keto-PGF-1-alpha. Low levels were sustained up to 72 hours in neonates who received 3 doses of ibuprofen, whereas subsequent re-increases were observed at 72 hours after only 1 dose of ibuprofen.
Cutaneous use
Ibuprofen gel, specially formulated for external application, penetrates through the skin rapidly and extensively (approximately 22% of a finite dose within 48 hours), achieving high, therapeutically relevant local concentrations in underlying soft tissues, joints and the synovial fluid, whilst producing plasma levels that are unlikely to be sufficient to cause any systemic side-effects, other than in rare individuals who are hypersensitive to ibuprofen. Furthermore, there do not appear to be any appreciable differences between the oral and topical routes of administration regarding metabolism or excretion.
The medicated plaster provides a topical formulation of ibuprofen designed to provide a sustained transfer of ibuprofen through the skin directly to the local site of the pain and inflammation.
In a human pharmacokinetic study, 28 subjects had the medicated plaster applied once daily for 5 consecutive days over a 7 day observation period. Plasma concentrations of ibuprofen rose rapidly reaching a mean concentration of 0.49 (95% CI: 0.39-0.58) micro grams/ml 24hr after application of the first patch. On day 5 of treatment, the mean Cmax was 0.51 (95% CI: 0.44-0.60) micro grams/ml, and the mean AUC0-24 was 9.59 (95% CI: 8.33-11.0) micro grams hr/ml. The mean Cmax and systemic bioavailability are low compared to oral ibuprofen and consistent with literature reviews for topical NSAIDs. The typical Cmax for a 200-400mg counterpart oral dose of ibuprofen is in the order of 20-50 micro grams/ml. The low Cmax and low AUC for the medicated plaster indicate that if used concomitantly with systemic ibuprofen, the contribution of the medicated plaster to systemic ibuprofen exposure would be negligible.
The PK profile demonstrated that of ibuprofen does not accumulate on repeated application and that there is rapid attenuation to baseline within 24 hours after discontinuation.
Rectal administration
Orally given ibuprofen is partly absorbed in the stomach and completely absorbed from the small intestine. Following rectal administration, the active substance is almost completely absorbed, reaching plasma concentrations similar to those seen after oral ingestion. Peak plasma levels occur in one to two hours after oral administration. Ibuprofen is bound to plasma proteins to the extent of 99 per cent.
After metabolization in the liver (hydroxylation, carboxylation), the pharmacologically inactive metabolites are completely excreted, mainly through the kidneys (90 per cent) and partly in the bile. The elimination half-life is about two hours.
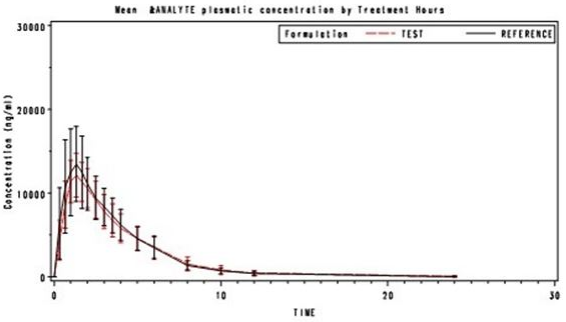
The following results were achieved in a bioavailability study performed in 2004 in 28 healthy volunteers, in comparison with a reference standard:
| Test preparation Ibuprofen 150 milligram sup positories (ibuprofen) | Reference standard Ibuprofen suspension 150 milligram (oral) | |
|---|---|---|
| Peak plasma concentration (Cmax, µg/ml): | 12.8 ± 2.6 | 16.6 ± 3.1 |
| Time of peak plasma concentration (tmax, hr): Area under the plasma concentration-time curve (AUC, µg/ml*hr): | 1.443 ± 0.455 | 1.285 ± 0.635 |
| 53.94 ± 14.85 | 56.25 ± 12.68 |
These values are expressed (AUC0-inf) is 95.50% in relation to the oral suspension (reference standard)
Average course of plasma concentrations in comparison with a reference standard in a concentration-time diagram:
Preclinical safety data
After systemic application, the subchronic and chronic toxicity of ibuprofen in animal experiments showed up mainly in form of lesions and ulcerations in the gastro-intestinal tract.
In vitro and in vivo studies gave no clinically relevant evidence of a mutagenic potential of ibuprofen. In studies in rats and mice no evidence of carcinogenic effects of orally applied ibuprofen was found.
Systemically applied ibuprofen inhibited ovulation in rabbits and led to implantation disorders in various animal species (rabbit, rat, mouse). Experimental studies in rat and rabbit have shown that ibuprofen crosses the placenta. Following administration of maternotoxic doses, an increased incidence of malformations (ventricular septal defects) occurred in the progeny of rats.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.